��Ŀ����
��Դ�����ǵ�ǰ����������ٵ�һ���ش���⣬H2��CO��CH3OH������Ҫ����Դ���ʣ����ǵ�ȼ��������Ϊ285.8 kJ/mol��282.5 kJ/mol��726.7 kJ/mol����ش�
(1)��֪CO��H2��һ�������¿��Ժϳɼ״���CO+2H2=CH3OH����H2��CO��Ӧ����CH3OH���Ȼ�ѧ����ʽΪ�� ��
(2)��ͼΪij��ȼ�ϵ�صĹ���ԭ��ʾ��ͼ��a��b��Ϊ���Ե缫��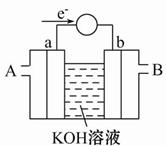
��ʹ��ʱ�������� ��ͨ��(�A����B��)��
�ڼ���ʹ�õġ�ȼ�ϡ��Ǽ״���a���ĵ缫��ӦʽΪ�� ________________
�ۼ���ʹ�õġ�ȼ�ϡ���ˮú��(�ɷ�ΪCO��H2)�����ֵ�ص��ͭ�����ƽ�������6.4 g�����������ı�״����ˮú�������Ϊ ��
(1)CO(g)+2H2(g)=CH3OH(l) ��H="-127.4" kJ/mol
(2)��B
��CH3OH -6e-+8OH-=CO32-+6H2O
��2.24 L
����

��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g)
FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g) CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g)
CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
(1)��500 ��ʱ���з�Ӧ��,CO2����ʼŨ��Ϊ2 mol��L-1,CO��ƽ��Ũ��Ϊ����������
(2)��Ӧ��Ϊ��������(ѡ����ȡ����ȡ�)��Ӧ��
(3)700 ��ʱ��Ӧ�ٴﵽƽ��״̬,Ҫʹ��ƽ�������ƶ�,������������ʱ,���Բ�ȡ�Ĵ�ʩ����������(�����)��
A.��С��Ӧ����� B.ͨ��CO2 C.�¶����ߵ�900 �� D.ʹ�ú��ʵĴ���
E.����Fe����
(4)����ͼ����Ϸ�Ӧ�ٵ�����������(�����)(ͼ��vΪ����,��Ϊ�������CO����,TΪ�¶���T1>T2)��
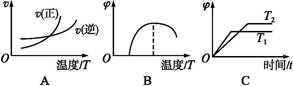
(5)�ɷ�Ӧ�ٺ͢ڿ����,��Ӧ2Fe(s)+O2(g)
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� (6)�����ø�˹����д��Fe(����)��O2(����)�����õ�Fe2O3(����)���Ȼ�ѧ����ʽ:����
��15�֣���Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]����֪��
��2NH3��g���� CO2��g���� NH2CO2NH4��s�� ��H �� -159.47 kJ��mol-1
��NH2CO2NH4��s���� CO(NH2)2��s���� H2O��g�� ��H �� +116.49 kJ��mol-1
��H2O��l���� H2O��g�� ��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ ��
��2����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�
CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L��1��H2��0.8mol��L��1��CH4��0.8mol��L��1��H2O��1.6mol��L��1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ �� ��CO2��ƽ��ת����Ϊ ��
������������ͬ�ĺ��ݾ��ȣ������û�������������ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g) ��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ���� ������ĸ����
| A������I��II������Ӧ������ͬ |
| B������I��II��CH4�����ʵ���������ͬ |
| C������I��CO2�����ʵ���������II�еĶ� |
| D������I��CO2��ת����������II��CH4��ת����֮��С��1 |
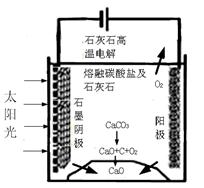
�������������̵�����ת����ʽ�� ��
��������ⷴӦ���¶�С��900��ʱ����̼����ȷֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ �������ĵ缫��ӦʽΪ ��
����һ�ֵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���塣
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
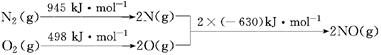
��2����֪��N2(g)+ O2(g)��2 NO(g) ��H����180 kJ ? mol-1
2NO(g)+2 CO(g)��N2(g) + 2 CO2(g) ��H����746 kJ ? mol-1
��ӦCO(g) + O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
��3����һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ�� N2(g)��3H2(g) 2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
��4���ڹ̶�������ܱ������У�1.0��103 kPaʱ��������Ӧ N2(g)+3H2(g) 2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 51 | K1 | K2 |
�� K1 K2�����������������������
�����и�����˵�������ϳɰ���Ӧһ���ﵽƽ��״̬���� ������ĸ����
a��������N2��H2��NH3��Ũ��֮��Ϊ1:3:2
b��NH3��Ũ�ȱ��ֲ���
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
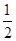 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��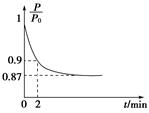
 2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��

 N2O4��g������H2����56.9 kJ��mol��1
N2O4��g������H2����56.9 kJ��mol��1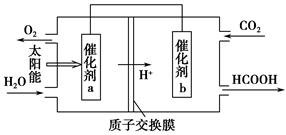
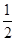 O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1