��Ŀ����
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ2NO��g����2CO��g�� 2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0���>����<������
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v��N2����________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c��CO2����T1��S2�����´ﵽƽ������еı仯���ߡ�
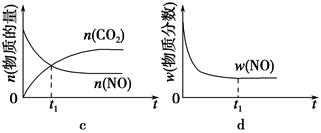
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________������ţ���
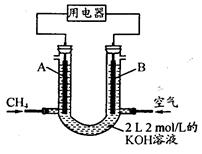
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
���磺CH4��g����2NO2��g��=N2��g����CO2��g����2H2O��g������H1����867 kJ��mol��1
2NO2��g�� N2O4��g������H2����56.9 kJ��mol��1
N2O4��g������H2����56.9 kJ��mol��1
д��CH4��g������ԭN2O4��g������N2��g����CO2��g����H2O��g�����Ȼ�ѧ����ʽ��__________________________________________________________________
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ_______________________________________��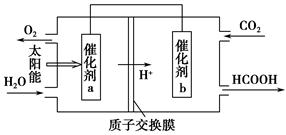
�۳����£�0.1 mol��L��1��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka��________��
��1����<����0.025 mol��L��1��s��1������ͼ
��bd
��2����CH4��g����N2O4��g��=N2��g����CO2��g����2H2O��g������H����810.1 kJ��mol��1
��CO2��2H����2e��=HCOOH
��10��7 mol��L��1
����

 ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�������ȼ��ʱ�ܷų��������ȣ���Ҳ��Һ��ʯ��������Ҫ�ɷ֣���Ϊ��ԴӦ�������ǵ��ճ������������֪��
��2C3H8(g) ��7O2(g) = 6CO(g)��8H2O(g) ��H = ��2389.8 kJ/mol
��2CO(g) + O2(g) = 2CO2(g) ��H = ��566 kJ/mol
��H2O(l) = H2O(g) ��H =" +" 44.0 kJ/mol
��1��д��C3H8ȼ��ʱȼ���ȵ��Ȼ�ѧ����ʽ ��
��2��C3H8�ڲ�������������ȼ�գ�����CO��CO2��H2O(g)�������еIJ���ͨ��һ������̶����ܱ�
�����У���һ�������·������¿��淴Ӧ�� CO(g) + H2O(g) CO2(g) + H2(g)
CO2(g) + H2(g)
�÷�Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�����¶�Ϊ800�棬�ڼס������������ܱ������У���ʼʱ�����±����ݽ���Ͷ�ϣ���ַ�Ӧֱ���ﵽƽ�⡣
| | H2O | CO | CO2 | H2 |
| �� ������/g�� | 1.8 | 8.4 | a | 1 |
| �� ������/g�� | 1.8 | 2.8 | 0 | 0 |
����ʼʱ��Ҫʹ�������з�Ӧ������Ӧ������У���a��ȡֵ��Χ�� ���ﵽƽ��
ʱ����������CO��ת����Ϊ ��
����ͼ��ʾ�����������з�Ӧ��t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ijһ�������������仯���������t2ʱ�̸ı������������ �� ��������Ҫ�㼴�ɣ���

��3��CO2����NaOH��Һ���յõ�Na2CO3��NaHCO3��
�� Na2CO3��Һ������Ũ���ɴ�С��˳��Ϊ���� ���������������� ������������
�� ��֪25��ʱ��H2CO3�ĵ���ƽ�ⳣ��K1 = 4.4��10-7 mol/L��K2 = 4.7��10-11 mol/L����Na2CO3��Һ��pHΪ11ʱ�� ��Һ��c(HCO3-)��c(CO32-) = ��
�� 0.1 mol/L Na2CO3��Һ��c(OH-) �� c(H+ ) = [�ú�c(HCO3��)��c(H2CO3)�ķ��ű�ʾ]��
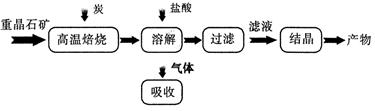
ij������ȼ���ɼס��������л����϶��ɣ��ס����������ʺ���C��H��O����Ԫ���е����ֻ����֡���֪�ס��Ҽ�CO��H2��ȼ�������£�
| ���� | �� | �� | CO | H2 |
| ȼ����/(kJ��mol��1) | 1 366 | 5 518 | 283 | 286 |
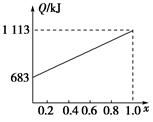
ȡ�ס��Ұ���ͬ������ϵ�ȼ��23 g����������O2��ȼ��ʱ���ų�������Q���������ҵ����ʵ�������x�Ĺ�ϵ��ͼ��ʾ������
(1)�ҵ���Է�������Mr(��)��________��
(2)160 g�ɼס����Ե����ʵ�����϶��ɵ�ȼ����347.2 L O2��ǡ����ȫȼ�գ���492.8 L���壬��ȴ������ʱ����ʣ��224 L(����������ڱ�״���²ⶨ)���ɴ˿���û�����У�C��H��O��ԭ�Ӹ�����Ϊ________���ס��ҵķ���ʽΪ����________����________��
(3)1 mol�ɼס����Ե����ʵ�����϶��ɵ�ȼ����һ������O2��ȼ�գ��ų�����2 876 kJ����Ӧ������CO________mol��
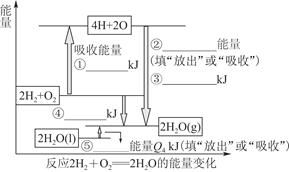
 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ�� O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1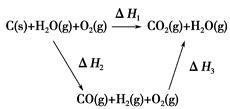

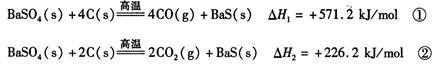
 2CO(g)�ġ�H = kJ/mol
2CO(g)�ġ�H = kJ/mol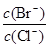 = ��[��֪��
= ��[��֪��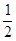 O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1
O2(g)��CO(g)��2H2(g) ��H2=��35.4 kJ��mol-1 [Cu(NH3)3]Ac��CO ��H��0
[Cu(NH3)3]Ac��CO ��H��0