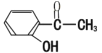
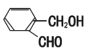
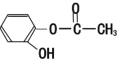
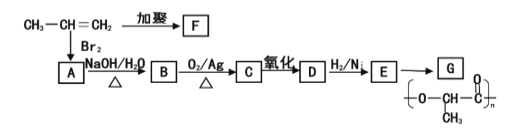
��Ŀ����
����Ŀ��CO��ɫ��ζ�ж����������ÿ����в�������CO�ж���ʧȥ������һ��CO�����ǵĹ���ԭ����ͼ��ʾ����װ���е����Ϊ�����ƣ������ƣ�����O2�������ڹ������NASICON�������ƶ�������˵���д������ (����)��

A.�����ĵ缫��ӦʽΪCO��O2����2e��=CO2
B.����ʱ�缫b��������O2���ɵ缫a��缫b�ƶ�
C.����ʱ�����ɵ缫aͨ������������缫b
D.��������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
���𰸡�B
��������
A.ȼ�ϵ����ȼ��������ʧȥ���ӱ�������A����ȷ��
B.ԭ����е�����е����������ƶ���O2����b�缫����缫a��B�����
C.ԭ��ع���ʱ�����ɸ��������·����������C����ȷ��
D.������λʱ�������ĵ�һ����̼�࣬β����һ����̼�����ߣ�D����ȷ��
��ѡB��

����Ŀ�������ҵ���������Ժ�����ˮ�Ի�������Ⱦ������������Cr(��)����Ҫ��Ⱦ��ɲ��ö��ִ������������ȥ���������Ͽ�֪��
�������Ի����£�Cr(��)ͨ����Cr2O72-����ʽ���ڣ�
��Cr2O72-����������ǿ��CrO42-��
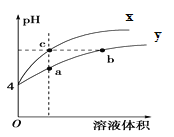
�۳����£�����������������������ʽ����ʱ��Һ��pH���±���ʾ��
������ | Fe3�� | Fe2�� | Cr3�� |
��ʼ������pH | 1.9 | 7.0 | 4.3 |
������ȫ��pH | 3.2 | 9.0 | 5.6 |
��.��ʴ��ط�
(1)�����Ժ�����ˮ��Ͷ�ŷ���м�ͽ�̿������ԭ���ԭ����ԭCr(��)�����й��ڽ�̿��˵����ȷ����_______(����ĸ����)��
a����ԭ��ص������� b���ڷ�Ӧ������ԭ����c��������������ݲ���
��.��ԭ��
�����Ժ�����ˮ�м�������NaCl���壬��FeΪ�缫��⣬����һ��ʱ�䣬��Cr(OH)3��Fe(OH)3���������ų����Ӷ�ʹ��ˮ�и����������ŷű������װ����ͼ1��ʾ��
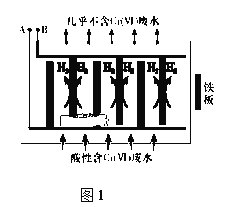
(2)A�����ӵ�Դ��______________����A���Ϸ����ĵ缫��ӦʽΪ_______________
(3)��ʼʱ��B���ϳ��˷�������H2�ķ�Ӧ�⣬��������Cr2O72-��B����ֱ�ӷŵ磬�÷�Ӧ�ĵ缫��ӦʽΪ_________________
(4)�������У���Һ��pH��ͬʱ��ͨ��ʱ��(t)����Һ�и�Ԫ��ȥ���ʵĹ�ϵ��ͼ2��ʾ��
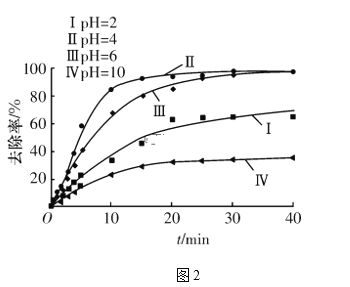
����ͼ��֪����ԭ��Ӧ��ȡ�����pH��ΧΪ____(����ĸ����)��
a��2��4������������b��4��6������������c��6��10
�ڽ������ߢ�����ߢ�ȥ���ʵ͵�ԭ��________________