ƒøƒ⁄»ð
°æƒø°ø”…”⁄√æ∫œΩæþ”–”≤∂»¥Û°¢√Ð∂»–°°¢…¢»»–‘∫√°¢øπ’–‘∫√µ»”≈“Ï–‘ƒÐÀ¸±ª”√”⁄÷∆± º«±æµÁƒ‘Õ‚ø«°¢æ∫»¸◊‘––≥µ≥µºÐµ»°£œ÷≥∆»°“ª∂®÷ ¡øµƒ√次∫œΩ—˘∆∑∑≈»Î500 mLœ°¡ÚÀ·÷–£¨πÃû´≤ø»ÐΩ‚≤¢∑≈≥ˆ∆¯Ã°£¥˝∑¥”¶ÕÍ»´∫Û£¨œÚÀ˘µ√»Ð“∫÷–º”»ÎNaOH»Ð“∫£¨…˙≥…≥¡µÌµƒŒÔ÷ µƒ¡ø”κ”»ÎNaOH»Ð“∫µƒÃª˝πÿœµ»Áœ¬ÕºÀ˘ æ°£

(1)∫œΩ÷–Alµƒ÷ ¡øŒ™__________________°£
(2)NaOH»Ð“∫µƒŒÔ÷ µƒ¡ø≈®∂»Œ™__________________°£
(3)œ°¡ÚÀ·µƒŒÔ÷ µƒ¡ø≈®∂»Œ™__________________°£
°æ¥∞∏°ø5.4g 4.0 mol/L 0.8 mol/L
°æΩ‚Œˆ°ø
£®1£©∏˘æðÕºœÛø…÷™£¨0°´25 mL∑¢…˙À·ºÓ÷–∫Õ£¨25°´200 mL∑¢…˙¿Î◊””κӅ˙≥…≥¡µÌµƒ∑¥”¶£¨200°´240mL∑¢…˙Al(OH)3+NaOH=NaAlO2+2H2O£¨200 mL ±…˙≥…≥¡µÌ◊Ó∂ý£¨»Ð“∫÷–µƒ»Ð÷ Œ™¡ÚÀ·ƒ∆£¨”…ÕºœÛø…÷™£¨«‚—ıªØ√浃ŒÔ÷ µƒ¡øŒ™0.15 mol£¨»ÐΩ‚µƒ«‚—ıªØ¬¡µƒŒÔ÷ µƒ¡øŒ™£∫0.35 mol-0.15 mol=0.2 mol£¨∏˘æ𬡑≠◊” ÿ∫„ø…µ√Alµƒ÷ ¡ø£ª
£®2£©∏˘æð∑¥”¶Al(OH)3+NaOH=NaAlO2+2H2Oø…«Ûµ√«‚—ıªØƒ∆µƒŒÔ÷ µƒ¡ø≈®∂»£ª
£®3£©»Ð“∫◊Ó∫Ûµƒ»Ð÷ Œ™¡ÚÀ·ƒ∆£¨∏˘æð‘™Àÿ ÿ∫„ø…÷™£¨n(H2SO4) = n(SO42-) = n(Na+)/2 = n(NaOH)/2£¨æð¥À∑÷Œˆ◊˜¥°£
£®1£©√次∫œΩ—˘∆∑∑≈»Î500 mLœ°¡ÚÀ·÷–£¨πÃû´≤ø»ÐΩ‚…˙≥…√æ¿Î◊”°¢¬¡¿Î◊”£¨œÚ¥À»Ð“∫÷–º”»ÎNaOH»Ð“∫£¨¥”…˙≥…≥¡µÌÕºœÛ∑÷Œˆø…÷™£¨«‚—ıªØƒ∆ê˝¥”0-25 mL ±√ª”–≥¡µÌ…˙≥…£¨Àµ√˜‘≠»Ð“∫÷–¥Ê‘⁄«‚¿Î◊”º¥¡ÚÀ·π˝¡ø£¨200-250 mL∂Œ≥¡µÌ≤ø∑÷œ˚ ߣ¨∑¢…˙µƒ∑¥”¶ «£∫Al(OH)3+OH-=AlO2-+2H2O£¨”…¬¡‘™Àÿ ÿ∫„µ√£∫n(Al) = n[Al(OH)3] = (0.35-0.15) mol = 0.2 mol£¨m(Al)= 0.2mol °¡27g/mol = 5.4 g£¨
π ¥∞∏£∫5.4 g£ª
£®2£©n(Al) = n[Al(OH)3] = n(OH-) = n(NaOH) = (250-200) mL°¡10-3°¡c(NaOH) = 0.2mol£¨c(NaOH)= 4.0 mol/L£ª
£®3£©‘⁄200 mL ±£¨≥¡µÌ ««‚—ıªØ√æ∫Õ«‚—ıªØ¬¡£¨»Ð÷ Œ™¡ÚÀ·ƒ∆£¨¥À ±V(NaOH)=200 mL£¨n(NaOH)=0.2 L°¡4.0 mol/L=0.8 mol£¨‘Ún(H2SO4) = n(SO42-) = n(Na+)/2= n(NaOH)/2 = 0.4 mol£¨c(H2SO4)= 0.4 mol/0.5 L=0.8 mol/L°£

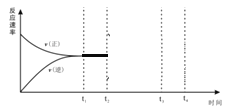
°æƒø°øΩ´“ª∂®¡øµƒSO2(g)∫ÕO2(g)∑÷±Õ®»Îê˝Œ™2Lµƒ∫„»ð√б’»ð∆˜÷–£¨‘⁄≤ªÕ¨Œ¬∂»œ¬Ω¯––∑¥”¶£∫2SO2(g)+ O2(g)![]() 2SO3 °˜H<0°£µ√µΩ»Á±Ì÷–µƒ¡Ω◊È ˝æ𣨜¬¡–Àµ∑®≤ª’˝»∑µƒ «
2SO3 °˜H<0°£µ√µΩ»Á±Ì÷–µƒ¡Ω◊È ˝æ𣨜¬¡–Àµ∑®≤ª’˝»∑µƒ «
µ—ȱý∫≈ | Œ¬∂»/°Ê | ∆Ω∫‚≥£ ˝/mol-1°§L | ∆ º¡ø/mol | ∆Ω∫‚¡ø/mol | ¥ÔµΩ∆Ω∫‚À˘–Ë ±º‰/min | ||
SO2 | O2 | SO2 | O2 | ||||
1 | T1 | K1 | 4 | 2 | x | 0.8 | 6 |
2 | T2 | K2 | 4 | 2 | 0.4 | y | t |
A. T1°¢T2µƒπÿœµ£∫T1 £æ T2
B. x= 1.6£¨y=0.2 £¨t<6
C. K1°¢K2µƒπÿœµ£∫K2£æK1
D. µ—È1‘⁄«∞6minµƒ∑¥”¶ÀŸ¬ ¶‘(SO2)=0.2 mol°§L-1°§min-1
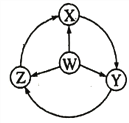
°æƒø°ø»ÁÕºW°¢X°¢Y°¢ZŒ™Àƒ÷÷ŒÔ÷ £¨»Ùº˝Õ∑ «ƒÐ“ª≤Ω◊™ªØµƒ≥£º˚∑¥”¶£¨∆‰÷–≥£Œ¬œ¬ƒÐΩ¯––µƒ «£® £©
—°œÓ | W | X | Y | Z |
|
A | S | SO2 | SO3 | H2SO4 | |
B | Al | AlCl3 | NaAlO2 | Al2(SO4)3 | |
C | Fe | FeCl3 | Fe(OH)2 | FeCl2 | |
D | Na | Na2O2 | NaOH | NaCl |
A. A B. B C. C D. D