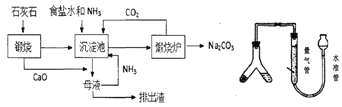
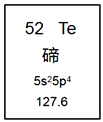
��Ŀ����
����Ŀ�������ǻ������������ֺ������绢���������������ִ���ҵ��ζ����������ϡ�����ȶ��ģ������������ܽ���Ũ����������![]() ��
��
(1)��д����������Ũ���ᷴӦ�����ӷ���ʽ��________________________
(2)����![]() ʱ�����ɷ���������
ʱ�����ɷ���������![]() ��д���÷�Ӧ�Ļ�ѧ����ʽ��_________
��д���÷�Ӧ�Ļ�ѧ����ʽ��_________
(3)![]() �ǽ�ǿ������������������ڵ�Ũ�������ò������������з�Ԫ�ر���ԭΪ��ɫ��
�ǽ�ǿ������������������ڵ�Ũ�������ò������������з�Ԫ�ر���ԭΪ��ɫ��![]() ��д���÷�Ӧ�����ӷ���ʽ��________________________
��д���÷�Ӧ�����ӷ���ʽ��________________________
(4)![]() �������������ǿ�Ӧ���ɷ����Σ�������Ϊ
�������������ǿ�Ӧ���ɷ����Σ�������Ϊ![]() ��������ǿ�����ɺ���������
��������ǿ�����ɺ���������![]() ���Ρ���д��
���Ρ���д��![]() �ֱ����ռ���Һ��ϡ���ᷴӦ���ɵ��εĻ�ѧʽ��
�ֱ����ռ���Һ��ϡ���ᷴӦ���ɵ��εĻ�ѧʽ��
______________________
(5)��ҵ���ýӴ���������ʱҪ�õ�![]() ��������
��������![]() �Ĺ����У�450��ʱ����
�Ĺ����У�450��ʱ����![]() ��
��![]() ֮���ת����
֮���ת����![]() ˵��
˵��![]() �ڽӴ�������������������������________________________
�ڽӴ�������������������������________________________
���𰸡�V+6H++5NO3-=VO2++5NO2��+3H2O2NH4VO3![]() V2O5+2NH3��+H2OV2O5+6H++2Cl-
V2O5+2NH3��+H2OV2O5+6H++2Cl-![]() 2VO2++Cl2��+3H2ONa3VO4 (VO2)2SO4�����������ã�
2VO2++Cl2��+3H2ONa3VO4 (VO2)2SO4�����������ã�
��������
(1)�����������ܽ���Ũ����������VO2+���ʽ�������Ũ���V+6H++5NO3-=VO2++5NO2��+3H2O������������������ǣ�V+6H++5NO3-=VO2++5NO2��+3H2O��
(2)������������NH4VO3ʱ�����ɷ���������V2O5���䷽��ʽΪ��2NH4VO3![]() V2O5+2NH3��+H2O������������������ǣ�2NH4VO3
V2O5+2NH3��+H2O������������������ǣ�2NH4VO3![]() V2O5+2NH3��+H2O��
V2O5+2NH3��+H2O��
(3)Ũ������V2O5��Ӧ��������Ԫ�صĻ��ϼ����ߣ���Ԫ�ر���ԭΪ��ɫ��VO2+���䷴Ӧ���ӷ���ʽΪ��V2O5+6H++2Cl-![]() 2VO2++Cl2��+3H2O������������������ǣ�V2O5+6H++2Cl-
2VO2++Cl2��+3H2O������������������ǣ�V2O5+6H++2Cl-![]() 2VO2++Cl2��+3H2O��
2VO2++Cl2��+3H2O��
(4)V2O5��ǿ�Ӧ����Na3VO4��V2O5��ǿ������(VO2)2SO4������������������ǣ�Na3VO4��(VO2)2SO4��
(5)�ɷ���ʽV2O5+SO2=2VO2+SO3��4VO2+O2=2V2O5��֪��˵��V2O5�ڽӴ�������������������ã�����������������ǣ������������ã���