��Ŀ����
6���ߴ�������[��ѧʽ��Sr��NO3��2]���������źŵơ���ѧ�����ȣ���1����ҵ���������г���������ơ����ᱵ�����ʣ���������ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ���ᣮ���������Ϣ������ᴿ�����ȵ�ʵ�鲽�裮
��ȡ�����ʵ���������Ʒ�������м���ŨHNO3�����裮
�ڹ��ˣ�����ŨHNO3ϴ������
�۽���������ˮ�У����Թ�������ʹBa2+���������ú�����£�N2H4�����������ỹԭ������pH=7��8�����ˣ�
�ܽ���Һ���������pH=2��3������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�
�ݽ��õ���Sr��NO3��2•2H2O������100�������¸���õ��ߴ������ȣ�
��2��Sr��NO3��2�����ֽ⣬����Sr��NO2��2��O2����500��ʱSr��NO2��2��һ���ֽ�����SrO���������ȡ һ��������Sr��NO2��2��Sr��NO3��2��Ʒ����������ȫ�ֽ⣬�õ�5.20g SrO�����5.08g������壮�������Ʒ��Sr��NO3��2������������д��������̣�
���� ��1���������к�������ơ����ᱵ�����ʣ�������ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ���ᣬ����ȼ���Ũ���ᣬ�ܽ�����ƣ����˺��ټ����������ʹBa2+���������˺�������Ũ����Һ�ɵ������ȣ�
��2���������ʵ�������������غ㶨����ʽ���㣮
��� �⣺��1���������к�������ơ����ᱵ�����ʣ�������ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ���ᣬ����ȼ���Ũ���ᣬ�ܽ�����ƣ����˺��ټ����������ʹBa2+���������˺�������Ũ����Һ�ɵ������ȣ���
��Ӧ�ȼ���Ũ���ᣬ�Գ�ȥ����ƣ��ʴ�Ϊ�������м���ŨHNO3��
�ڽ������Һ����룬�ù��˵ķ����ɳ�ȥ����ƣ�����Ũ����ϴ�ӳ������ʴ�Ϊ�����ˣ�����ŨHNO3ϴ��������
�ܽ���������Һ����Ũ������ȴ�ᾧ�ɵõ������Ⱦ��壬�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��2��m����Ʒ��=5.20 g+5.08 g=10.28 g��
n��SrO��=$\frac{5.20g}{104g•mo{l}^{-1}}$=0.05 mol��
n[Sr��NO3��2]��212 g•mol-1+n[Sr��NO2��2]��180 g•mol-1=10.28 g��
n[Sr��NO3��2]+n[Sr��NO2��2]=0.05 mol��
��ã�n[Sr��NO3��2]=0.04 mol��
n[Sr��NO2��2]=0.01 mol��
w[Sr��NO3��2]=$\frac{0.04mol��212g•mo{l}^{-1}}{10.28g}$��100%=82.49%��
����Ʒ��Sr��NO3��2����������Ϊ82.49%��
���� ���⿼�����ʵķ��롢�ᴿ���ۺϲ������漰ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬Ϊ��Ƶ���㣬ע�����ʵ���ԭ���Ͳ�������Ŀ�ѶȲ���

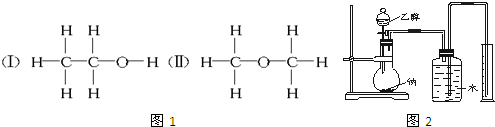
Ϊ�ⶨ��ṹ��Ӧ�������ʵ������Խ��ж��ԡ�����ʵ�飮�ָ����Ҵ����ơ�ˮ����Ҫ����������ס��ҡ���������λͬѧֱ��������ͼ2����װ�ÿ�ʼ����ʵ��ȷ���Ҵ��Ľṹ��
��1��ѧ���õ�һ��ʵ�����ݣ�
| �Ҵ������ʵ���/mol | ���������/L |
| 0.10 | 1.12����״���� |
��2��ͬѧ�ҷֱ�ȷ����4.60g �Ҵ����ж��ʵ�飬����������ſ���Ͳ�ڵ������Ϊ���ɵ�H2���������ɱ�״����С��1.12L�����������Ͳ��������ͬѧ������ɵ�����ô����Ϊ������������Ʒ�к�������ˮ��ɵģ�����Ϊ��ȷ�𣿲���ȷ�����ȷ������ȷ�������������Ϊ��ȷ����˵�����ɣ��������Ϊ����ȷ���Dz������������ԭ��Ӧ����ʲô�����ƿ����Ͳ֮�䲣��������ˮ�������û��������
��3��ͬѧ����Ϊʵ��ɹ��Ĺؼ��У���װ��������Ҫ���ã���ʵ�鿪ʼǰȷȷ���Ҵ������������������ܹ��ƿ��ˮ�������������������IJ��㷽����ȷ������ȷ��������ȷ���Т٢ڢۢݣ�������ţ�
��4��ͬѧ������ͨ�������Ҵ���������ȷ���Ҵ���������ô������Ҫ֪���������������Ҵ���Ʒ���ܶȺ������
| A�� | ����������������ȵ���ԭ�ӷ���${��}_{1}^{1}$H | |
| B�� | ��ϩ�ı���ģ�ͣ� | |
| C�� | ˫��ˮ�ĵ���ʽ��${H}_{•}^{•}{\stackrel{••}{\underset{••}{O}}}_{•}^{•}{\stackrel{••}{\underset{••}{O}}}_{•}^{•}$H | |
| D�� | ������Ľṹʽ��H-O-Cl |
| A�� | Ca��HCO3��2��Һ�е������NaOH��Һ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| B�� | ��NaAlO2��Һ��ͨ�������CO2��CO2+2H2O+AlO2-�TAl��OH��3��+HCO3- | |
| C�� | 0.01 mol•L-1 ��NH4Al��SO4��2��Һ��0.02 mol•L-1��Ba��OH��2��Һ�������ϣ�Al3++2SO42-+NH4++2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3•H2O | |
| D�� | ��Ca��ClO��2��Һ��ͨ�������SO2��ClO-+SO2+H2O�THClO+HSO3- |
| A�� | �÷�Ӧ�д���������ӵõ��ӣ����ֻ�ԭ�� | |
| B�� | �÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��1 | |
| C�� | �÷�Ӧÿ����3 molX��ת�Ƶ��ӵ����ʵ���Ϊ2mol | |
| D�� | ��������ͭ��Һ������������Һ�������ڱڲ����γɶƲ� |
| A�� | ����ԭ�������ĵ�����ԭ���������������Ǵ�1��8�ظ����� | |
| B�� | Ԫ�ص���������ԭ�������ĵ������������Ա仯 | |
| C�� | ����ԭ�������ĵ���������Ԫ�صĻ��ϼ۳��������Եı仯 | |
| D�� | Ԫ�����ʵ������Ա仯�ǽ�ָԭ�Ӱ뾶�������Ա仯 |
| A�� | �״����Ҷ��� | B�� | ���������Ҷ��� | C�� | ���ѡ��Ҵ� | D�� | �״���2-���� |
| A�� | ���ú�ˮ������ѧ�仯�����Ƶ�ʳ�κ͵�ˮ | |
| B�� | ����װ�ľ��������������ˮ������Ч�����շ������ͷŵļ�ȩ�������к����� | |
| C�� | ����������ϩ�;���ϩȼ��ʱ������ϩ�����Ķ�����̼�� | |
| D�� | ʯ�ͷ���ɻ�����ᡢ���� |