��Ŀ����
����Ŀ����1����ͬ���ʵ������ơ�þ�������������ᷴӦʱ�ų������������Ϊ__________����ͬ�������ơ�þ�������������ᷴӦʱ�ų��������������_________���ֱ�Ϊ0.3 mol���ơ�þ����Ͷ��100 mL 1 mol��L-1��������Һ�У����߲������������______________��
��2����һ�������Ƶ��������ڳ���CO2���壬Ȼ��������ע��������NaOH��Һ�������ý����ܷ�ڡ�����һ��ʱ��ޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹�������
�ޱ��ڰ������ԭ����______________________�����ӷ���ʽ����Ϊ____________________________��
���ٹ����ԭ����___________________________�����ӷ���ʽ����Ϊ___________________________��
���𰸡�1��2��3 ![]() ��
��![]() ��
��![]() 3��2��2 CO2�����ն���������ѹѹ�� 2OH-+CO2=CO32-+H2O ��������Ӧ����H2��ѹǿ��������¹��� 2Al+2OH-+2H2O=2AlO2-+3H2��
3��2��2 CO2�����ն���������ѹѹ�� 2OH-+CO2=CO32-+H2O ��������Ӧ����H2��ѹǿ��������¹��� 2Al+2OH-+2H2O=2AlO2-+3H2��
��������
��1����ͬ���ʵ������ơ�þ�������������ᷴӦ��������ȫ��Ӧ�����ݵ���ת���غ����֪���������������֮�ȵ��ڽ����ṩ�������ʵ���֮�ȣ����������������֮��Ϊ1:2:3��
�������Ϊ1g����![]() ��
��![]() ��
��![]() ��������ȫ��Ӧ�������������֮�ȵ��ڽ����ṩ�������ʵ���֮�ȣ��������������֮��Ϊ
��������ȫ��Ӧ�������������֮�ȵ��ڽ����ṩ�������ʵ���֮�ȣ��������������֮��Ϊ![]() ��
��
![]() ������Һ��
������Һ��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ����:
����:![]()
![]()
![]()
![]()
![]()
![]() ��
��
��Al��Mg����������![]() ���㣬��Al��Ӧ��������Ϊ0.1mol����Mg��Ӧ��������Ϊ0.1mol�������ƻ��ã�����ˮ��Ӧ������������
���㣬��Al��Ӧ��������Ϊ0.1mol����Mg��Ӧ��������Ϊ0.1mol�������ƻ��ã�����ˮ��Ӧ������������![]() ����֪�����Ʒ�Ӧ��������Ϊ0.15mol�����ơ�����þ�������������֮��Ϊ
����֪�����Ʒ�Ӧ��������Ϊ0.15mol�����ơ�����þ�������������֮��Ϊ![]() ��
��
��2��
���������ڳ���CO2�����NaOH�ᷢ����Ӧ2NaOH+CO2=Na2CO3+H2O����ʹ��������ѹǿ��С��������������ѹ�������¶�����������NaOH���������ijɷ���������Ӧ������H2�������ķ�Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2�������Թ�����ѹǿ���������������ֻ����¹�������

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�����Ŀ����ͼ��ʾ��������γ�ʾ��ͼ������ͼʾ�ش��������⡣
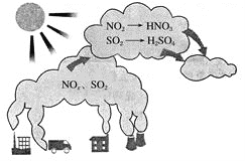
��1����������������������_____��
A��CO2 B��SO2 C��N2 D��NO2
��2��������ˮ��Ʒ1�ݣ�ÿ��һ��ʱ��ⶨ����ˮ��Ʒ��pH�������������£�
����ʱ��/h | 0 | 1 | 2 | 3 | 4 |
��ˮ��pH | 4.73 | 4.63 | 4.56 | 4.55 | 4.55 |
�������ݣ��ش��������⣺
����ˮ��Ʒ��pH�仯��ԭ����____(�û�ѧ����ʽ��ʾ)��
���������ȡ����������ˮ������ˮ���ϣ�pH����____��ԭ����_____(�û�ѧ����ʽ��ʾ)��
��3�����д�ʩ�У��ɼ������������;������____(����ĸ)��
������ú��ȼ�� �ڰѹ����̴���� ��ȼ������ �������ữ�������м�ʯ�� �ݿ�������Դ
A���٢ڢ� B���ڢۢܢ� C���٢ۢ� D���٢ۢܢ�