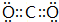��Ŀ����
�����ֳ���������Ԫ��A��B��C�����ǵ�ԭ��������������AԪ��ԭ��������������BԪ��ԭ��������������1����BԪ��ԭ��������������CԪ��ԭ��������������һ�룮�ס��ҡ����ֱ���A��B��CԪ����ۺ���������Σ��ס�����ҺpH��7������ҺpH��7����Ϊ��ɫ����ζ�����壬��Ϊ����ɫ���壮������֮�����Ӧ��ϵ���£���仯�صIJ�������ȥ����
�Իش�
��1��CԪ�ص�����Ϊ �����ĽṹʽΪ ����ĵ���ʽΪ ��
��2��д����Ӧ�ٵ����ӷ���ʽ�� ��
��3��д���ܵĻ�ѧ����ʽ�� ��
��4����ҵ�Ͽ���B�ĵ�����C������������ˮ����M������������Լ��Ʊ��������Ʊ�1mol��������ҪM�����ʵ���Ϊ ��

�Իش�
��1��CԪ�ص�����Ϊ
��2��д����Ӧ�ٵ����ӷ���ʽ��
��3��д���ܵĻ�ѧ����ʽ��
��4����ҵ�Ͽ���B�ĵ�����C������������ˮ����M������������Լ��Ʊ��������Ʊ�1mol��������ҪM�����ʵ���Ϊ
���������������Ϣ��֪����Ϊ����ɫ�����붡���巴Ӧ�ƶ�ΪNa2O2����ΪCO2������Һ�ͱ���Һ������Ӧ���ɶ�����̼���壬�ס��ҡ����ֱ���A��B��CԪ����ۺ���������Σ��ס�����ҺpH��7������ҺpH��7��˵����ΪNa2CO3��NaHCO3����+��=�ף��жϼ�ΪNa2CO3����ΪNaHSO4��������Ԫ��A��B��C�����ǵ�ԭ��������������AԪ��ԭ��������������BԪ��ԭ��������������1����BԪ��ԭ��������������CԪ��ԭ��������������һ�룬AΪC��BΪAl��CΪS������ҺpH��7����ΪNaAlO2������ҺΪAl2��SO4��3����ΪAl��OH��3�����ƶϳ������ʺ�Ԫ�ط����ش����⣮
����⣺��Ϊ����ɫ�����붡���巴Ӧ�ƶ�ΪNa2O2����ΪCO2������Һ�ͱ���Һ������Ӧ���ɶ�����̼���壬�ס��ҡ����ֱ���A��B��CԪ����ۺ���������Σ��ס�����ҺpH��7������ҺpH��7��˵����ΪNa2CO3��NaHCO3����+��=�ף��жϼ�ΪNa2CO3����ΪNaHSO4��������Ԫ��A��B��C�����ǵ�ԭ��������������AԪ��ԭ��������������BԪ��ԭ��������������1����BԪ��ԭ��������������CԪ��ԭ��������������һ�룬AΪC��BΪAl��CΪS������ҺpH��7����ΪNaAlO2������ҺΪAl2��SO4��3����ΪAl��OH��3��
��1�����ݷ����жϣ�CԪ��Ϊ��Ԫ�أ���Ϊ������̼����Ϊ�������ƣ����Զ�����̼�ṹʽΪ��O=C=O���������Ƶ���ʽΪ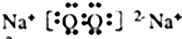 ��
��
�ʴ�Ϊ����O=C=O��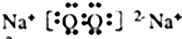 ��
��
��2����Ӧ����ƫ�����ƺ������������Ʒ�Ӧ�����������������ƺ��ǣ���Ӧ�����ӷ���ʽΪ��AlO2-+4H+=Al3++2H2O��
�ʴ�Ϊ��AlO2-+4H+=Al3++2H2O��
��3����Ӧ������������̼������Һ������˫ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪ��3Na2CO3+Al2��SO4��3+2H2O=2Al��OH��3��+3CO2��+3Na2SO4��
�ʴ�Ϊ��3Na2CO3+Al2��SO4��3+2H2O=2Al��OH��3��+3CO2��+3Na2SO4��
��4����ҵ�Ͽ���BΪAl�ĵ�����CΪ�������������ˮ����MΪH2SO4��������������Լ��Ʊ���ΪAl��OH��3����Ӧ�Ļ�ѧ����ʽΪ��
2Al+3H2SO4=Al2��SO4��3+3H2����2Al+2H2O+2NaOH=2NaAlO2+3H2����Al2��SO4��3+6NaAlO2 +12H2O=8Al��OH��3��+3Na2SO4�����Ʊ�1molAl��OH��3������ҪH2SO4�����ʵ���Ϊ��
3H2SO4��Al2��SO4��3��6NaAlO2��8Al��OH��3
3 8
n 1mol
n=0.375mol��
�ʴ�Ϊ��0.375mol��
��1�����ݷ����жϣ�CԪ��Ϊ��Ԫ�أ���Ϊ������̼����Ϊ�������ƣ����Զ�����̼�ṹʽΪ��O=C=O���������Ƶ���ʽΪ
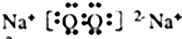 ��
���ʴ�Ϊ����O=C=O��
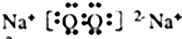 ��
����2����Ӧ����ƫ�����ƺ������������Ʒ�Ӧ�����������������ƺ��ǣ���Ӧ�����ӷ���ʽΪ��AlO2-+4H+=Al3++2H2O��
�ʴ�Ϊ��AlO2-+4H+=Al3++2H2O��
��3����Ӧ������������̼������Һ������˫ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪ��3Na2CO3+Al2��SO4��3+2H2O=2Al��OH��3��+3CO2��+3Na2SO4��
�ʴ�Ϊ��3Na2CO3+Al2��SO4��3+2H2O=2Al��OH��3��+3CO2��+3Na2SO4��
��4����ҵ�Ͽ���BΪAl�ĵ�����CΪ�������������ˮ����MΪH2SO4��������������Լ��Ʊ���ΪAl��OH��3����Ӧ�Ļ�ѧ����ʽΪ��
2Al+3H2SO4=Al2��SO4��3+3H2����2Al+2H2O+2NaOH=2NaAlO2+3H2����Al2��SO4��3+6NaAlO2 +12H2O=8Al��OH��3��+3Na2SO4�����Ʊ�1molAl��OH��3������ҪH2SO4�����ʵ���Ϊ��
3H2SO4��Al2��SO4��3��6NaAlO2��8Al��OH��3
3 8
n 1mol
n=0.375mol��
�ʴ�Ϊ��0.375mol��
���������⿼�������Pת����ϵ�ķ����жϣ��������ʺͷ�Ӧ������ԭ�ӽṹ����Һ���ʵķ����ǽ���ؼ�����Ҫ�������仯�������ʵ�Ӧ�ã���Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ

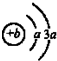 ��2012?�ɶ�ģ�⣩����X��Y��Z���ֳ���������Ԫ�أ�x��ԭ�ӽṹʾ��ͼ��ͼ��Y��ZΪͬ���ڽ���Ԫ�أ�Y��Z������������Ӧˮ������Է�Ӧ�����κ�ˮ���ش��������⣺
��2012?�ɶ�ģ�⣩����X��Y��Z���ֳ���������Ԫ�أ�x��ԭ�ӽṹʾ��ͼ��ͼ��Y��ZΪͬ���ڽ���Ԫ�أ�Y��Z������������Ӧˮ������Է�Ӧ�����κ�ˮ���ش��������⣺ �Իش�
�Իش�