��Ŀ����
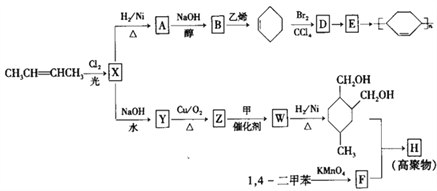
����Ŀ����ϩ��Ϊԭ�ϣ��ϳ�ijЩ�߾����·�����£�
��֪�� ����д��
�����![]() ��
��
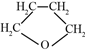
��1��CH3CH��CHCH3��������____________��
��2��X�к��еĹ�������______________��
��3��A��B�Ļ�ѧ����ʽ��_____________��
��4��D��E�ķ�Ӧ������_______________��
��5����Ϊ����F����NaHCO3��Ӧ����CO2��
�������й�˵����ȷ����_______________��
a���л���Z�ܷ���������Ӧ b���л���Y��HOCH2CH2OH��Ϊͬϵ��
c���л���Y�ķе��B�� d���л���F���뼺�������۳ɾۺ���
��Y��ͬ���칹���ж��֣�д�����ӽṹ�к�������������ͬ���칹���������_____��
��Z��W�Ļ�ѧ����ʽ��__________________��
��6���߾���H�Ľṹ��ʽ��_________________��
���𰸡� 2����ϩ ̼̼˫������ԭ�� ClCH2CH2CH2CH2Cl+2NaOH![]() CH2=CH-CH=CH2+2NaCl+2H2O ��ȥ��Ӧ ad 4
CH2=CH-CH=CH2+2NaCl+2H2O ��ȥ��Ӧ ad 4 ![]()
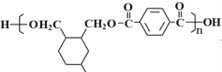
��������������Ҫ�����л���Ľṹ�����ʡ�
��1��CH3CH��CHCH3��������2����ϩ��
��2��X��ȡ�����X�к��еĹ�������̼̼˫������ԭ����
��3��A��B�����ȶ������ȥ��Ӧ����Ӧ�Ļ�ѧ����ʽ��ClCH2CH2CH2CH2Cl+2NaOH![]() CH2=CH-CH=CH2+2NaCl+2H2O��
CH2=CH-CH=CH2+2NaCl+2H2O��
��4��D��E����±��������ȥ��Ӧ����Ӧ��������ȥ��Ӧ��
��5����Ϊ����F����NaHCO3��Ӧ����CO2��
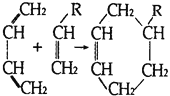
��a���л���Z�Ǵ��������IJ������Z����ȩ�����ܷ���������Ӧ����a��ȷ��b���л���Y���Ӻ��еĹ�������̼̼˫����һ���ǻ�����HOCH2CH2OH�Ĺ����Ų���ȫ��ͬ���������Dz�Ϊͬϵ���b����c��Y�����ǻ�������֮������γ������B��ϩ����B�в���������������л���Y�ķе��B�ߣ���c����d���л���F�Ƕ�Ԫ���ᣬ���뼺�������۳ɾۺ����d��ȷ����ѡad��
��Y���Ӻ���̼̼˫���������ǻ������ӽṹ�к�������������ͬ���칹��ֱ��Ǽ����������������������������������������4�֡�
��Z��W����ϩ���ļӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽ��![]() ��
��
��6���߾���H�Ƕ�������������۲��H�Ľṹ��ʽ��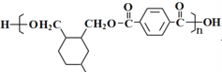 ��
��