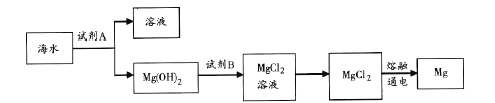
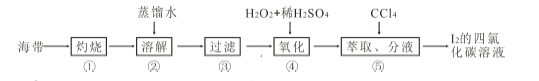
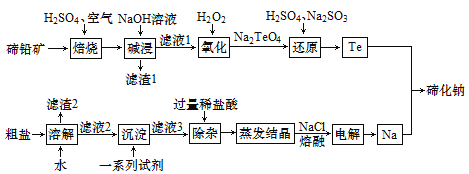
��Ŀ����
����Ŀ��ʵ�������ܶ�Ϊ1.25g��mL��1����������36.5%��Ũ��������0.1mol��L��1������240mL����ش��������⣺
(1)Ũ��������ʵ���Ũ��Ϊ_______��
(2)����240 mL0.1mol��L��1������Ӧѡ��_______mL������ƿ����Ҫ��ȡŨ�������Ϊ_______mL��
(3)����ʱ�������ձ���������������ƿ�⣬����Ҫ�IJ���������_______��
(4)��ȷ�IJ���˳����________������ţ�
���ý�ͷ�ι���μ�ˮ��ʹ��Һ��Һ��ǡ��������ƿ�̶�������
��������ƿ�м�ˮ��Һ��ӽ�ƿ���ϵĿ̶���1-2cm��
�۸��ݼ��㣬����Ͳ��ȡһ�������Ũ����
�ܽ�����ƿ�ǽ�����ҡ��
�ݽ�Ũ���ᵹ���ձ��м�ˮϡ�ͣ��������ò��������裬���ô�����ȴ
����Һ�ò���������ע���©��������ƿ��
������ˮϴ���ձ���������2��3�Σ�����ÿ�ε�ϴ��ҺҲע������ƿ��
(5)���в�����������Һ��Ũ�ȴ�С�к�Ӱ�� (����ƫ��������ƫС��������Ӱ����)��
�ٶ���ʱ�����ӿ̶��ߣ�Ũ��_________��
�ڶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ���ˮ���̶��ߣ�Ũ��________��
���𰸡�12.5mol/L 250 2.0 ��ͷ�ι� �ۢݢޢߢڢ٢� ƫ�� ƫС
��������
(1)����c=![]() ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ�
(2)����������Һ���ѡ����ʹ�������ƿ��������Һϡ��ǰ���������ʵ����ʵ������������ҪŨ����������
(3)����������Һ�IJ���ȷ��ʹ�õ�������
(4)����������Һ�IJ����жϲ���˳��
(5)����c=![]() ������������
������������
(1)�������ʵ���Ũ����������������ʽ����֪�ܶ�Ϊ1.25gmL-1����������36.5%��Ũ�������ʵ���Ũ��c=![]() mol/L=12.5 mol/L��
mol/L=12.5 mol/L��
(2)����240mL0.1mol/L������Ӧѡ��250mL����ƿ��ʵ������250mL��Һ������ҪŨ��������ΪV��������Һϡ��ǰ���������ʵ����ʵ��������V��12.5mol/L=250mL��0.1mol/L�����V=2.0ml��
(3)����һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ��ڶ���ʱ�������ձ���������������ƿ�⣬����Ҫ�IJ��������ǽ�ͷ�ιܣ�
(4)����һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ʲ���˳�����Ϊ�ۢݢޢߢڢ٢ܡ�
(5)�ٶ���ʱ�����ӿ̶��ߣ�������Һ���ƫС�������Ƶ���ҺŨ��ƫ��
�ڶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ���ˮ���̶��ߣ����²�������������ʵ����ʵ���ƫС��ʹ���Ƶ���ҺŨ��ƫС��

����Ŀ��H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
��.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ________��
(3)��֪��25 ��ʱ��Ksp(SnS)��1.0��10��25��Ksp(CdS)��8.0��10��27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2����ʼ����ʱ����Һ��c(Cd2��)��________(��Һ����仯���Բ���)��
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)��H2(g) ![]() H2S(g)��CO(g)����H����7 kJ��mol��1��
H2S(g)��CO(g)����H����7 kJ��mol��1��
��.CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H����42 kJ��mol��1��
CO2(g)��H2(g)����H����42 kJ��mol��1��
(4)��֪������1 mol�����еĻ�ѧ���������յ����������ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/(kJ��mol��1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
����x��________��
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g)����������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶�(T)�Ĺ�ϵ��ͼ��ʾ��
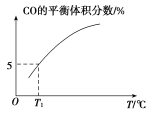
�������¶����ߣ�CO��ƽ���������_____(����������������С��)��ԭ��Ϊ_______
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶��£�COS��ƽ��ת����Ϊ_____����Ӧ����ƽ�ⳣ��Ϊ_____(������λ��Ч����)��