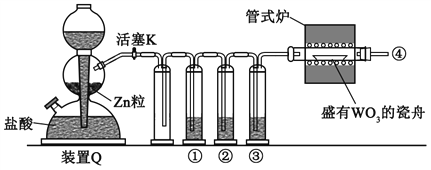
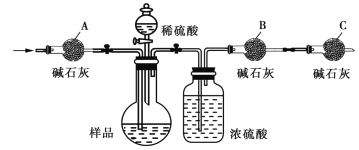
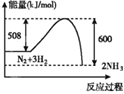
��Ŀ����
����Ŀ������Ҫ����գ�
(1)��ͼ���ʾ�����H2�ӳɺ�IJ����У�������̼ԭ��(�����ĸ���ͬ��ԭ�ӻ�ԭ����)����Ϊ__________��
(2)ij�߷��ӽṹ��ʽΪ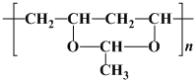 ��������
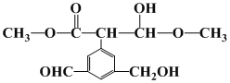
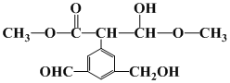
��������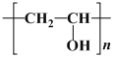 ����һ����X�����Ϸ�Ӧ�õ��ġ���֪���Ϲ�������H2O���ɣ�����X�Ľṹ��ʽ����Ϊ________________
����һ����X�����Ϸ�Ӧ�õ��ġ���֪���Ϲ�������H2O���ɣ�����X�Ľṹ��ʽ����Ϊ________________
(3)1mol����C��H��O����Ԫ�ص��л���A��ϡ������ˮ������1molB��1molC��B������N(C)��N(H)��4��5��135��Mr(B)��140��C��B������̼ԭ������ͬ����Mr(B)��Mr(C)��2
��B�ķ��Ӿ��и߶ȶԳ��ԣ������ϵ�һ��ȡ����ֻ��һ�֡�B�������Na��Ӧ��������NaOH��Ӧ��д��B�Ľṹ��ʽ_________________________��
��C�Ƕ�λ��Ԫȡ���ķ����廯���д��A�Ľṹ��ʽ_____________________��
���𰸡�4 CH3CHO ![]()
![]()
��������
(1)  ������H2�ӳɺ�IJ���Ϊ
������H2�ӳɺ�IJ���Ϊ ����ȷ������̼ԭ�ӣ�
����ȷ������̼ԭ�ӣ�
(2) ij�߷��ӽṹ��ʽΪ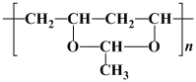 ��������
�������� ����һ����X�����Ϸ�Ӧ�õ��ģ������Ϲ�������H2O���ɣ���ϸ߷��ӵĽṹ��ԭ���غ��ƶ�X�Ľṹ��ʽ��
����һ����X�����Ϸ�Ӧ�õ��ģ������Ϲ�������H2O���ɣ���ϸ߷��ӵĽṹ��ԭ���غ��ƶ�X�Ľṹ��ʽ��
(3)�л���A��ϡ������ˮ������1molB��1molC��B�ķ��Ӿ��и߶ȶԳ��ԣ������ϵ�һ��ȡ����ֻ��һ�֣�˵��B�ı�������1��Hԭ�ӣ���B��ȡ����Ӧ��ͬ���ҷ��ӶԳƣ�B�������Na��Ӧ��������NaOH��Ӧ��˵��B�к���-OH��BΪ������CΪ���B������N(C)��N(H)��4��5��135��Mr(B)��140����B�ķ���ʽΪ��C4H5��xOy��B�����к�������x���ٵ���2�����135<53x+16y<140��xֻ��Ϊ2��������y=2����B�ķ���ʽΪC8H10O2����B�Ľṹ��ʽΪ![]() ��B����Է�������Ϊ138��Mr(B)=Mr(C)+2����C����Է�������Ϊ136��C��B��̼ԭ������ͬ��CӦ��B��2��H��C�ķ���ʽΪC8H8O2��C�Ƕ�λ��Ԫȡ���ķ����廯�������1��Ϊ-COOH������һ��Ϊ-CH3��C�Ľṹ��ʽΪ
��B����Է�������Ϊ138��Mr(B)=Mr(C)+2����C����Է�������Ϊ136��C��B��̼ԭ������ͬ��CӦ��B��2��H��C�ķ���ʽΪC8H8O2��C�Ƕ�λ��Ԫȡ���ķ����廯�������1��Ϊ-COOH������һ��Ϊ-CH3��C�Ľṹ��ʽΪ![]() ����AΪ
����AΪ![]() ���Դ˽����⡣
���Դ˽����⡣
(1)  ������H2�ӳɺ�IJ���Ϊ
������H2�ӳɺ�IJ���Ϊ �����ӽṹ�е�����̼ԭ��Ϊ
�����ӽṹ�е�����̼ԭ��Ϊ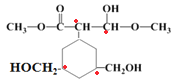 ������4������̼ԭ�ӣ�
������4������̼ԭ�ӣ�
(2)ij�߷��ӽṹ��ʽΪ ��������
�������� ����һ����X�����Ϸ�Ӧ�õ��ģ������Ϲ�������H2O���ɣ��ɸ߷��ӵĽṹ��ԭ���غ��֪�ǻ��ϵ���ԭ�Ӻ�X�����ڵ���ԭ�ӽ�ϳ�H2O����X�Ľṹ��ʽΪCH3CHO��
����һ����X�����Ϸ�Ӧ�õ��ģ������Ϲ�������H2O���ɣ��ɸ߷��ӵĽṹ��ԭ���غ��֪�ǻ��ϵ���ԭ�Ӻ�X�����ڵ���ԭ�ӽ�ϳ�H2O����X�Ľṹ��ʽΪCH3CHO��
(3) �л���A��ϡ������ˮ������1molB��1molC��B�ķ��Ӿ��и߶ȶԳ��ԣ������ϵ�һ��ȡ����ֻ��һ�֣�˵��B�ı�������1��Hԭ�ӣ���B��ȡ����Ӧ��ͬ���ҷ��ӶԳƣ�B�������Na��Ӧ��������NaOH��Ӧ��˵��B�к���-OH��BΪ������CΪ���B������N(C)��N(H)��4��5��135��Mr(B)��140����B�ķ���ʽΪ��C4H5��xOy��B�����к�������xspan>���ٵ���2�����135<53x+16y<140��xֻ��Ϊ2��������y=2����B�ķ���ʽΪC8H10O2����B�Ľṹ��ʽΪ![]() ��B����Է�������Ϊ138��Mr(B)=Mr(C)+2����C����Է�������Ϊ136��C��B��̼ԭ������ͬ��CӦ��B��2��H��C�ķ���ʽΪC8H8O2��C�Ƕ�λ��Ԫȡ���ķ����廯�������1��Ϊ-COOH������һ��Ϊ-CH3��C�Ľṹ��ʽΪ
��B����Է�������Ϊ138��Mr(B)=Mr(C)+2����C����Է�������Ϊ136��C��B��̼ԭ������ͬ��CӦ��B��2��H��C�ķ���ʽΪC8H8O2��C�Ƕ�λ��Ԫȡ���ķ����廯�������1��Ϊ-COOH������һ��Ϊ-CH3��C�Ľṹ��ʽΪ![]() ����AΪ
����AΪ![]() ��
��
�������Ϸ�����֪B�Ľṹ��ʽΪ![]() ��
��
��C�Ƕ�λ��Ԫȡ���ķ����廯���1��Ϊ-COOH������һ��Ϊ-CH3���ṹ��ʽΪ![]() ����A�Ľṹ��ʽΪ
����A�Ľṹ��ʽΪ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�