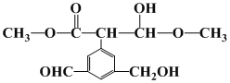
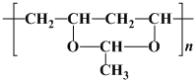
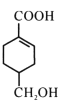
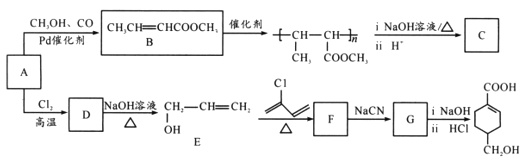
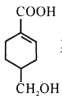
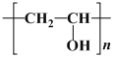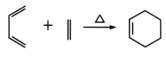��Ŀ����
����Ŀ��ijNa2CO3��Ʒ�л���һ������Na2SO4(��������ᾧˮ)��ijʵ��С��������·����ⶨ��Ʒ��Na2CO3������������
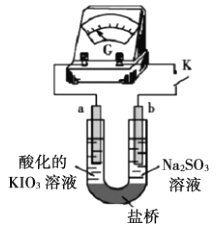
��1����ͬѧͨ���ⶨ������̼���������ⶨ̼���Ƶ�����������ʵ��װ����ͼ��
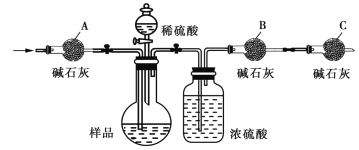
����Ҫʵ�鲽���У�a����װ����ͨ�������b�����������B��װ���ʯ�ҵ���������c����Һ©��������ʹϡ��������Ʒ��ַ�Ӧ�������IJ�����_____(���ظ�)��
�ڰ�����������ҵ��������A��������____�������C��������______��
��2����ͬѧ����ͼ��������������������װһ��װ�����Na2CO3���������IJⶨ��������Ʒ�ѳ�����ϣ�����װ��CO2���������е�Һ�塣

�٢���ʢװ����____(�����)��
A��Ũ���� B������NaHCO3��Һ C��10mol��L1���� D��2mol��L1����
���������Ӷ�Ӧ�ӿڵķ�ʽ�ǣ�A��___��B��__��C��___(����ӿڵı��)��
���ڲ����������ʱ����������뢤װ����ȸ�Ϊȷ����Ҫԭ����____�������������ڢ�װ�õ���һ���ŵ���______��
���𰸡�abcab ��ȥ�����еĶ�����̼ ��ֹ�����е�ˮ�ֺͶ�����̼�������B���� D D E F ��������������Ҳ����������������������û�� Һ�����˳������
��������
��1��ϡ��������Ʒ��Ӧ����������̼��Ȼ�����Ķ�����̼ͨ��װ�� B �ļ�ʯ���У������������仯���ݴ˽��м���̼��������������ʵ�������Ҫ��������Ҫʹ���ɵĶ�����̼ȫ������B�У����Ҫ���������ͬʱ��Ҫ��ֹװ���ں�����ˮ������������̼���� B �У�����Aͨһ��ʱ���������ȥװ���ڵ� CO2 ��ˮ������������ͨ����ʹ���ɵĶ�����̼ȫ������װ�� B�У�C��ֹװ���ں�����ˮ������������̼���� B �У�
��2�������������������������ӷ�ʽ���£�
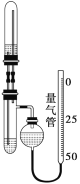
����ʢװ��Ӧ��������̼���Ʒ�Ӧ���ɶ�����̼��Һ�壬Ӧ������ѡ��ѡC����������Ũ��̫���ӷ����ڲ����������ʱ�������������������װ���뢤��ȸ�Ϊȷ���������������װ�õ����巢�������൱��һ����ѹװ�ã�Һ�����˳�����£����ҵ��µ�������û�м��������������С��ʵ����
��1���ٷ�Ӧǰ����װ����ͨ���������ȥװ���еĶ�����̼����ʱҪ��������B����ʢ��ʯ�ҵ���������Ȼ�����ʹ��Ʒ���Ὺʼ��Ӧ����ַ�Ӧ����ͨ�����ʹ��Ӧ���ɵĶ�����̼���屻�����B������գ�������һ�¸����B�����������������Ӧ���ɵĶ�����̼����������һ�������Ʒ��̼���Ƶ������������ʲ���˳��Ϊ�� abcab��
�ڰ�����������ҵ��������A�������dz�ȥ�����еĶ�����̼ �������C�������Ƿ�ֹ�����е�ˮ�ֺͶ�����̼�������B���ա�
��2�������������������������ӷ�ʽ���£�

�٢���ʢװ��Ӧ��������̼���Ʒ�Ӧ���ɶ�����̼��Һ�壬Ӧ������ѡ��ѡC����������Ũ��̫���ӷ�����ѡD��
�ڸ���ʵ��ԭ���ķ������������Ӷ�Ӧ�ӿڵķ�ʽ�ǣ�A��D��B��E��C��F��
���ڲ����������ʱ�������������������װ���뢤��ȸ�Ϊȷ���������������װ�õ����巢�������൱��һ����ѹװ�ã�Һ�����˳�����£����ҵ��µ�������û�м��������������С��ʵ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����͵���ڷ��ٷ�չ����Ϣ�����з�����Խ��Խ��Ҫ�����á�Li2FeSiO4�Ǽ��߷�չDZ������������ӵ�ص缫���ϣ���ƻ���ļ��������͵IJ�Ʒ���Ѿ�����һ���̶ȵ�Ӧ�á�����һ���Ʊ�Li2FeSiO4�ķ���Ϊ�����෨��2Li2SiO3+FeSO4![]() Li2FeSiO4+Li2SO4+SiO2
Li2FeSiO4+Li2SO4+SiO2
ijѧϰС�鰴����ʵ�������Ʊ�Li2FeSiO4���ⶨ���ò�Ʒ��Li2FeSiO4�ĺ�����
ʵ��(һ)�Ʊ����̣�
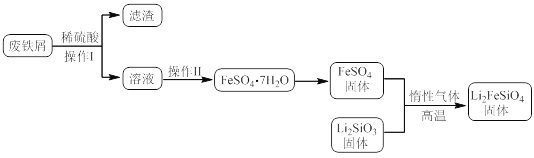
ʵ��(��)Li2FeSiO4�����ⶨ��
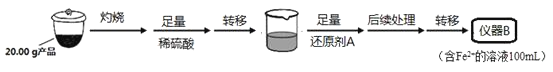
������B��ȡ20.00mL��Һ����ƿ�У���ȡ0.2000mol��L��1������KMnO4����Һװ������C�У���������ԭ�ζ����ⶨFe2+��������ط�ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�����ʲ�������KMnO4����Һ��Ӧ����4�εζ���ÿ������KMnO4��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ��� | 20.00mL | 19.98mL | 21.38mL | 20.02mL |
��1��ʵ��(��)�е��������ƣ�����B__������C__��
��2���Ʊ�Li2FeSiO4ʱ�����ڶ��������Χ�н��У���ԭ����__��
��3��������IJ���__���ڲ�����ʱ�������õ��IJ��������У�������ͨ©�����ձ��⣬����__��
��4����ԭ��A����SO2��д���÷�Ӧ�����ӷ���ʽ__����ʱ������������ҪĿ����__��
��5���ζ��յ�ʱ����Ϊ__�����ݵζ��������ȷ����Ʒ��Li2FeSiO4����������Ϊ_��
����Ŀ��ijС��̽��![]() ��Һ��
��Һ��![]() ��Һ�ķ�Ӧԭ����
��Һ�ķ�Ӧԭ����
��ʵ��һ���������۵�![]() ��Һ����
��Һ����![]() ������Һ(����)�У���Ϻ�Լ5���������Ա仯�������������ɫ���ֲ�Ѹ�ٱ�����
������Һ(����)�У���Ϻ�Լ5���������Ա仯�������������ɫ���ֲ�Ѹ�ٱ�����
(1)��Һ������˵��![]() ����__________�ԡ�
����__________�ԡ�
(2)�������ף�
��Ӧ��![]() ��
��
��Ӧ��![]() _____=_____+_____ �Ͽ�
_____=_____+_____ �Ͽ�
��Ӧ��![]() ��
��
д�����������£���Ӧ������ӷ���ʽ__________��
(3)��ʵ��һ������ɫ��Һ�м�������![]() ��Һ����ɫѸ����ȥ�����ֱ���ɫ���ݴ˵ó�
��Һ����ɫѸ����ȥ�����ֱ���ɫ���ݴ˵ó�![]() �����Ա�
�����Ա�![]() ǿ���ý���______(����������������������)��������_________��
ǿ���ý���______(����������������������)��������_________��
(4)Ϊ�˽�һ���о�![]() ��Һ��
��Һ��![]() ��Һ�ķ�Ӧԭ�����������ʵ�顣
��Һ�ķ�Ӧԭ�����������ʵ�顣
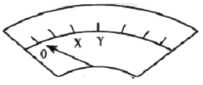
��ʵ�����װ����ͼ��ʾ��![]() �պϺ�������ָ��ƫת�����¼�����
�պϺ�������ָ��ƫת�����¼�����
���� |
|
| ||
ʱ��/min |
|
|
| |
ƫתλ�� | ��ƫ����Y���� | ָ��ص���0�������ַ�����X�������������������������� | ָ����� | |
��![]() �պϺ���b��������Һ���ڷŵ����
�պϺ���b��������Һ���ڷŵ����![]() ��ʵ�������__________��
��ʵ�������__________��
��![]() ʱ��ֱ����a�����μӵ�����Һ����Һδ������ȡa��������Һ���Թ��У��μӵ�����Һ����Һ�������ж�
ʱ��ֱ����a�����μӵ�����Һ����Һδ������ȡa��������Һ���Թ��У��μӵ�����Һ����Һ�������ж�![]() ��a���ŵ�IJ�����__________��
��a���ŵ�IJ�����__________��
(5)���й�������ʵ����ͺ�������__________(����ĸ���)��
A.ʵ��һ�У�5���������Ա仯����������Ϊ��Ӧ��Ļ��̫С����Ӧ����̫��
B.ʵ����У�ָ��ص���0��������������Ϊ��Ӧ��ȷ�Ӧ��죬����![]() ����
����![]() ������Ӧ
������Ӧ
C.ʵ����У��ַ�����X��������������Ϊ�����˷�Ӧ�������γ���ԭ���