��Ŀ����
14��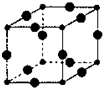 Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã�
Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã���1����N3-������ͬ����������ԭ�ӷ��ӵĿռ乹����V�Σ�
��2��Cu�������õĵ��硢���Ⱥ���չ�ԣ������Cu���е����Ե�ԭ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��sp2��sp3����ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�����ڡ��������ڡ���С�ڡ�����
��4��Cu+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d10������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2OCu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ���Ի������м��Եķ��ӵĽṹʽ
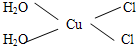 ��
����6��Cu3N�ľ����ṹ��ͼ��N3-����λ��Ϊ6��Cu+�뾶Ϊa pm��N3-�뾶Ϊb pm��Cu3N���ܶ�$\frac{103��1{0}^{30}}{4��a+b��^{3}{N}_{A}}$g/cm3���������ӵ���Ϊ������NA��ʾ��
���� ��1����N3-������ͬ����������Ϊ�ȵ����壬��NO2-���ȵ�����ṹ���ƣ����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��2���������ɵ��ӵĽ��������ܵ��磻
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ����ݴ��ж�̼ԭ�ӵ��ӻ���ʽ��̼ԭ���ӻ���ʽ��ͬ��������Dz�ͬ��
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ����д���̬���Ӻ�������Ų�ʽ��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���
��5��[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�
��6��Cu3N�ľ����ṹ��ͼ���������=12��$\frac{1}{4}$=3��С�����=$\frac{1}{8}$=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6��Cu3N���ܶȸ��ݦ�=$\frac{m}{V}$���㣮
��� �⣺��1����N3-������ͬ����������Ϊ�ȵ����壬��NO2-���õ�����ṹ���ƣ��������������Nԭ�Ӽ۲���ӶԸ���=2+$\frac{1}{2}$����5+1-2��2��=3�Һ���һ���µ��Ӷԣ�����ΪV�νṹ��
�ʴ�Ϊ��V�Σ�
��2��ͭ���ڽ������壬�����к��п��������ƶ��ĵ��ӣ�ͨ������ƶ��������ܵ��磬
�ʴ�Ϊ��CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
��3����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ���ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ��ǣ�
�ʴ�Ϊ��sp3��sp2�����ڣ�
��4��Cu+�ĺ�����28�����ӣ����ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10��ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ���Cu+��3d�����ȫ�����ȶ���
�ʴ�Ϊ��1s22s22p63s23p63d10�� Cu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ���
��5����[Cu��H2O��4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ��[Cu��H2O��2��Cl��2]���м��Եķ��ӣ�˵���÷��ӵĽṹ���Գƣ�����ṹʽΪ��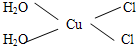 ��
��
�ʴ�Ϊ��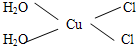 ��
��
��6��Cu3N�ľ����ṹ��ͼ���������=12��$\frac{1}{4}$=3��С�����=8��$\frac{1}{8}$=1�����Դ����ʾCuԭ�ӡ�С���ʾNԭ�ӣ�N3-����λ��=3��2=6�����������=[��2a+2b����10-10cm]3��Cu3N���ܶȦ�=$\frac{m}{V}$=$\frac{\frac{64��3+14}{{N}_{A}}}{[��2a+2b����1{0}^{-10}]^{3}}$g/cm3=$\frac{103��1{0}^{30}}{4��a+b��^{3}{N}_{A}}$g/cm3��
�ʴ�Ϊ��6��$\frac{103��1{0}^{30}}{4��a+b��^{3}{N}_{A}}$g/cm3��
���� ���⿼�������ʽṹ�����ʣ��漰�ռ乹�͡����������ʡ���������Ų��������ļ����֪ʶ�㣬�����ܶȹ�ʽ���۲���ӶԻ������ۡ�����ԭ����֪ʶ�������������Щ֪ʶ�㶼�ǿ����ȵ㣬�ѵ��ǣ�6�������ļ��㣬��Ŀ�Ѷ��еȣ�

| A�� | ����ڱȽϣ�c��Na+����c��NH4+�� | |
| B�� | ���е�����Ũ�ȵĴ�С��ϵ�ǣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| C�� | ����ڵ������ϵ���Һ�У�c��Cl-��=c��NH4+��+c��Na+��+c��NH3•H2O�� | |
| D�� | ����м�������������Һ��ʹ��ҺpH=7����c��CH3COO-������Na+�� |
| A�� | ���ʵ��ܶ��������� | B�� | ���ʵ��۵�ͷе��������� | ||
| C�� | Cl2���Դ�KI��Һ���û���I2 | D�� | Br2���Դ�NaCl��Һ���û���Cl2 |
| A�� | ��ˮ����ƽ�⣺Br2+H2O?HBr+HBrO ����AgNO3��Һ����Һ��ɫ��dz | |
| B�� | �ϳ� NH3��Ӧ��Ϊ��� NH3�IJ��ʣ�������Ӧ��ȡ��Խϵ��¶ȵĴ�ʩ | |
| C�� | ��ѹ�ȳ�ѹ�����ںϳ�SO3�ķ�Ӧ | |
| D�� | ��CO��g��+NO2��g��?CO2��g��+NO��g�� ƽ����ϵ����ѹǿ��ʹ��ɫ���� |
| A�� | 2���һ����� | B�� | 2��3��3���������� | ||
| C�� | 2����-3-���� | D�� | 2��3-���һ�-1-��ϩ |
| A�� | 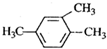 1��3��4-������ 1��3��4-������ | B�� | 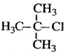 2-��-1-�ȱ��� 2-��-1-�ȱ��� | ||
| C�� | 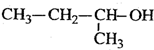 2-��-1-���� 2-��-1-���� | D�� | 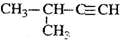 2-��-3-��Ȳ 2-��-3-��Ȳ |
| A�� | �٢� | B�� | �٢� | C�� | �� | D�� | ������ȷ |
| A�� | �ƾ���ˮ-���� | B�� | ����ˮ-��Һ | C�� | ʳ����ˮ-���� | D�� | KNO3��NaCl-���� |