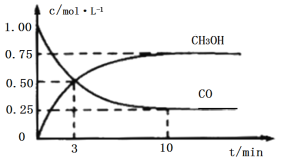
��Ŀ����
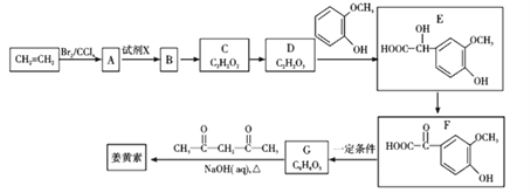
����Ŀ������Aֻ����������һ��ȡ����B��C��D��C�Ľṹ��ʽ����ͼ��  ��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ������ʾ��
��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ������ʾ��
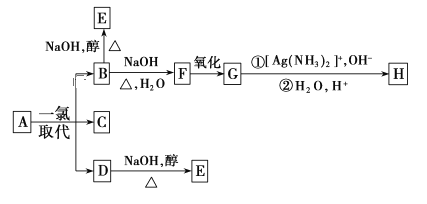
��ش��������⣺
(1)A�Ľṹ��ʽ��_________��
(2)H�Ľṹ��ʽ��________��
(3)Bת��ΪF�ķ�Ӧ����____��Ӧ(�Ӧ��������)��
(4)Bת��ΪE�ķ�Ӧ����_____��Ӧ(�Ӧ��������)��
(5)д���������ʼ�ת���Ļ�ѧ����ʽ��B��F��________����F��G��_______��
���𰸡�(CH3)3CCH2CH3 (CH3)3CCH2COOH )ȡ��(ˮ��) ��ȥ (CH3)3CCH2CH2Cl��H2O![]() (CH3)3CCH2CH2OH��HCl 2(CH3)3CCH2CH2OH��O2
(CH3)3CCH2CH2OH��HCl 2(CH3)3CCH2CH2OH��O2![]() 2(CH3)3CCH2CHO��2H2O
2(CH3)3CCH2CHO��2H2O
��������
����Aֻ����������һ��ȡ����B��C��D��C�Ľṹ��ʽ��![]() ����AΪ(CH3)3CCH2CH3��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E����EΪ(CH3)3CCH=CH2��B����ˮ�ⷴӦ����F��F������������������Ӧ����BΪ(CH3)3CCH2CH2Cl��DΪ(CH3)3CCH(Cl)CH3����FΪ(CH3)3CCH2CH2OH��GΪ(CH3)3CCH2CHO��HΪ(CH3)3CCH2COOH���ݴ˽��
����AΪ(CH3)3CCH2CH3��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E����EΪ(CH3)3CCH=CH2��B����ˮ�ⷴӦ����F��F������������������Ӧ����BΪ(CH3)3CCH2CH2Cl��DΪ(CH3)3CCH(Cl)CH3����FΪ(CH3)3CCH2CH2OH��GΪ(CH3)3CCH2CHO��HΪ(CH3)3CCH2COOH���ݴ˽��
����Aֻ����������һ��ȡ����B��C��D��C�Ľṹ��ʽ��![]() ����AΪ(CH3)3CCH2CH3��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E����EΪ(CH3)3CCH=CH2��B����ˮ�ⷴӦ����F��F������������������Ӧ����BΪ(CH3)3CCH2CH2Cl��DΪ(CH3)3CCH(Cl)CH3����FΪ(CH3)3CCH2CH2OH��GΪ(CH3)3CCH2CHO��HΪ(CH3)3CCH2COOH��
����AΪ(CH3)3CCH2CH3��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E����EΪ(CH3)3CCH=CH2��B����ˮ�ⷴӦ����F��F������������������Ӧ����BΪ(CH3)3CCH2CH2Cl��DΪ(CH3)3CCH(Cl)CH3����FΪ(CH3)3CCH2CH2OH��GΪ(CH3)3CCH2CHO��HΪ(CH3)3CCH2COOH��
(1)ͨ�����Ϸ���֪��A�ṹ��ʽΪ (CH3)3CCH2CH3��
(2)������������֪��H�Ľṹ��ʽ��(CH3)3CCH2COOH��
(3)Bת��ΪF��±����������ˮ�ⷴӦ��Ҳ����ȡ����Ӧ��
(4)Bת��ΪE��±������������ȥ��Ӧ��
(5)��B��F�Ļ�ѧ��Ӧ����ʽΪ��(CH3)3CCH2CH2Cl��H2O![]() (CH3)3CCH2CH2OH��HCl��
(CH3)3CCH2CH2OH��HCl��
��F��G�Ļ�ѧ��Ӧ����ʽΪ��2(CH3)3CCH2CH2OH��O2![]() 2(CH3)3CCH2CHO��2H2O��
2(CH3)3CCH2CHO��2H2O��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����Ŀ����֪ijNaOH�����к���NaCl���ʣ�Ϊ�ⶨ������ NaOH �������������������²���ʵ�飺
�� ���� 1.0g ��Ʒ����ˮ����� 250 mL ��Һ��
�� ȷ��ȡ 25.00 mL ������Һ����ƿ�У�
�� �μӼ��η�̪��Һ��
�� �� 0.10mol/L�ı�����ζ����Σ�ÿ����������������¼���£�
�ζ���� | ����Һ���(mL) | �����������Һ�����(mL) | |
�ζ�ǰ | �ζ��� | ||
1 | 25.00 | 0.50 | 20.60 |
2 | 25.00 | 6.00 | 26.00 |
3 | 25.00 | 1.10 | 21.00 |
��ش�
(1)����1.0g ��Ʒ����С�ձ���ҩ���⣬���õ�����Ҫ������_________��
(2)����Ʒ��� 250 mL ��Һ����С�ձ����������⣬�����õ��IJ���������_________��
(3)��_________�ζ���(������ʽ��������ʽ��)ʢװ 0.10mol/L �������Һ��
(4)�۲�ζ����յ�ʱ��Һ��ɫ�ı仯Ϊ_________��
(5)�ռ���Ʒ�Ĵ���Ϊ_________��
(6)����������������ⶨ���ƫ�ߵ���_________��
a���ζ�ǰ������ˮ��ϴ��ƿ
b��������ƿʱ������ƿ����Һ����
c�����ڵζ������в�����������Һ������ƿ��
d��ʢװ��Һ�ĵζ���ˮϴ��δ�ñ�Һ����ϴ
����Ŀ����3�ֲ�ͬ�����£��ֱ����ݻ�Ϊ2L�ĺ����ܱ������г���2molA��1molB��������Ӧ:2A(g)+B(g)![]() 2D(g)��H=QkJ/mol��������������ݼ��±�:
2D(g)��H=QkJ/mol��������������ݼ��±�:
ʵ���� | ʵ��I | ʵ��II | ʵ��III |
��Ӧ�¶�/�� | 700 | 700 | 750 |
��ƽ��ʱ��/min | 40 | 50 | 30 |
n(D)ƽ��/mol | 1.5 | 1.5 | 1 |
��ѧƽ�ⳣ�� | K1 | K2 | K3 |
����˵����ȷ����
A. �����¶��ܼӿ췴Ӧ���ʵ�ԭ���ǽ����˷�Ӧ�Ļ�ܣ�ʹ����Ӱٷ������
B. ʵ��III��ƽ����������������䣬����������ͨ��1molA��1molD��ƽ�ⲻ�ƶ�
C. ʵ��III��ƽ��������ڵ�ѹǿ��ʵ��1��9/10��
D. K3>K2>K1