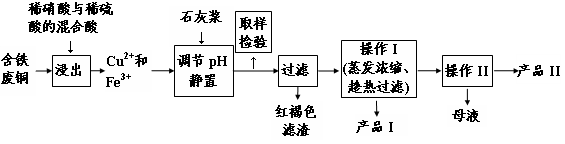
��Ŀ����
��ï�����ṹ��ͼ����һ�����͵Ľ����л������ʵ���ҳ����Ȼ������ͻ����ϩ�ڼ��������·�Ӧ�õ�����Ӧԭ��Ϊ��FeCl2+2C5H6+2KOH��Fe(C5H5)2+2KCl+2H2O
��ï�����۵�Ϊ172��173�棬��100�濪ʼ���������������ѡ����������ȷǼ����ܼ���������ˮ���Լ�ͷ����������ȶ����Ʊ��IJ������£�
����1����150mL������ƿ�м���25gϸ��ĩ״KOH��60mL��ˮ���ѣ�ͨ�뵪��������Լ10����ʹ֮�������ܽ⣬Ȼ�����5.5 mL�����ϩ,�ٽ���10���ӡ�
����2. ���ձ��м���25 mL����������6.5g���Ƶ���ˮ�Ȼ�����������40�沢����ʹ���ܽ⣬Ȼ������Һ©���С�
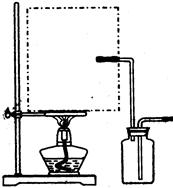
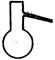
����3. ��ͼ��ʾ��װ��װ�����������Һ©���Ļ������������Ȼ������ȼ�������ƿ�У�����������1Сʱ��
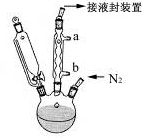
����4. ��Ӧ����������ﵹ��100mL 18%��������Һ�����ձ����ڱ�ԡ����ȴ������Լ10���ӣ�ʹ�ᾧ��ȫ��
����5. ���ˣ�����õIJ�Ʒ����ˮϴ��2��3�Σ����·�ɵõ����ƵĶ�ï����
��1������1��ͨ��N2��Ŀ�Ŀ����� ��
��2������2�У���Һ©����ߵIJ������ܵ������� ��
��3��ʵ��װ���У�������ͨˮ��ˮӦ�� �ڽ���ѡ��a��b����
��4������4����Ӧ��Ļ���ﵹ�������У�������Ҫ��Ӧ�����ӷ���ʽ�� ��
��5������5����ˮϴ������Ϊ ����˿ɲ��� �ķ�������һ���ᴿ��ï����

��ï�����۵�Ϊ172��173�棬��100�濪ʼ���������������ѡ����������ȷǼ����ܼ���������ˮ���Լ�ͷ����������ȶ����Ʊ��IJ������£�
����1����150mL������ƿ�м���25gϸ��ĩ״KOH��60mL��ˮ���ѣ�ͨ�뵪��������Լ10����ʹ֮�������ܽ⣬Ȼ�����5.5 mL�����ϩ,�ٽ���10���ӡ�
����2. ���ձ��м���25 mL����������6.5g���Ƶ���ˮ�Ȼ�����������40�沢����ʹ���ܽ⣬Ȼ������Һ©���С�
����3. ��ͼ��ʾ��װ��װ�����������Һ©���Ļ������������Ȼ������ȼ�������ƿ�У�����������1Сʱ��

����4. ��Ӧ����������ﵹ��100mL 18%��������Һ�����ձ����ڱ�ԡ����ȴ������Լ10���ӣ�ʹ�ᾧ��ȫ��
����5. ���ˣ�����õIJ�Ʒ����ˮϴ��2��3�Σ����·�ɵõ����ƵĶ�ï����
��1������1��ͨ��N2��Ŀ�Ŀ����� ��
��2������2�У���Һ©����ߵIJ������ܵ������� ��
��3��ʵ��װ���У�������ͨˮ��ˮӦ�� �ڽ���ѡ��a��b����
��4������4����Ӧ��Ļ���ﵹ�������У�������Ҫ��Ӧ�����ӷ���ʽ�� ��
��5������5����ˮϴ������Ϊ ����˿ɲ��� �ķ�������һ���ᴿ��ï����
��ÿ��2�֣���12�֣�
��1���ų�����ƿ�ڵĿ���
��2������©����ѹǿ������Һ��ų���3��b
��4��H+ + OH�� = H2O
��5������������� ��������
��1���ų�����ƿ�ڵĿ���
��2������©����ѹǿ������Һ��ų���3��b
��4��H+ + OH�� = H2O
��5������������� ��������
�õ����Ͽ�������Һ©����ߵIJ������ܵ��������ų�����ƿ�ڵĿ�����������Ӧ���½��ϳ�������Ӧ��Ļ���ﵹ�������з����кͷ�Ӧ������ˮϴ������Ϊ��ï�������������ü��������ķ����ᴿ��ï����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
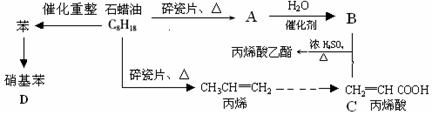
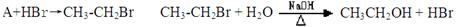

 2Fe2++I2����������Ϊ�������һ�����淴Ӧ��Fe3+��I����Ӧ�����Һ�����ɫ������I2����KI��Һ����ɫ��Ϊ̽�������ɫ��Һ���Ƿ�Fe3+������֤�����Ƿ���һ�����淴Ӧ��������ʵ���ҳ�����������Ʒ�������Լ���Ʒ�������дλ�ڴ�������±���0.1 mol/L��FeCl3��KI��KSCN��NaOH��H2SO4��KMnO4��Һ��CCl4������ˮ��
2Fe2++I2����������Ϊ�������һ�����淴Ӧ��Fe3+��I����Ӧ�����Һ�����ɫ������I2����KI��Һ����ɫ��Ϊ̽�������ɫ��Һ���Ƿ�Fe3+������֤�����Ƿ���һ�����淴Ӧ��������ʵ���ҳ�����������Ʒ�������Լ���Ʒ�������дλ�ڴ�������±���0.1 mol/L��FeCl3��KI��KSCN��NaOH��H2SO4��KMnO4��Һ��CCl4������ˮ��
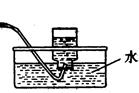


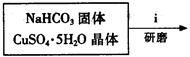
 Cl2����MnCl2��2H2O��
Cl2����MnCl2��2H2O��
 ��100�G��
��100�G��