��Ŀ����
����Ŀ��ʵʩ�Խ�Լ��Դ�ͼ��ٷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ����������ѧ֪ʶ���ش��������⣺
����֪��Ӧ����CH4(g)��H2O (g) ![]() CO(g)+3H2(g)����H��+206 kJ��mol��1
CO(g)+3H2(g)����H��+206 kJ��mol��1
��C(s)��H2O (g) ![]() CO(g)+ H2(g) ����H��+131 kJ��mol��1
CO(g)+ H2(g) ����H��+131 kJ��mol��1
(1)��ҵ��ȡ̿�ڵķ���֮һ�ǽ���������������ȵ�1300�������ѽ⡣��д�հס�CH4(g) ![]() C(s)+2H2(g)����H��________kJ��mol��1��
C(s)+2H2(g)����H��________kJ��mol��1��
(2)��800��ʱ����Ӧ�ٵ�ƽ�ⳣ��K1=1.0��ijʱ�̲�ø��¶��£��ܱ������и����ʵ����ʵ���Ũ�ȷֱ�Ϊ��c(CH4)=4.0 mol��L��1; c(H2O)=5.0 mol��L��1��c (CO)=1.5 mol��L��1��c(H2)=2.0 mol��L��1�����ʱ�ÿ��淴Ӧ��״̬��_______(�����)
a���ﵽƽ�� b��������Ӧ�����ƶ� c�����淴Ӧ�����ƶ�
���״���һ�ֿ�������Դ����ҵ����CO��H2���ϳɼ״���CO(g)+2H2(g) ![]() CH3OH(g)���ش��������⣺
CH3OH(g)���ش��������⣺
(1)һ�������£���CO��H2�����ʵ���֮��1��1���ں����ܱ������з������Ϸ�Ӧ��ƽ��ʱ������˵����ȷ����_______��
A��v(H2)��=v(CH3OH)�� B��2v(CO)=v(H2)
C��CO��H2ת������� D��CO��H2�����ʵ���֮�Ȳ��ٸı�
(2)ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ��¶�T1��T2��С��ϵ��T1____T2(������������������������)����Ӧ�¶ȵ�ƽ�ⳣ����С��ϵ��K1____K2(������������������������)��
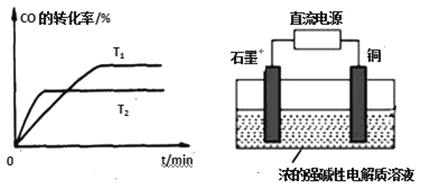
(3)�ü״�ȼ�ϵ����Ϊֱ����Դ�������ͼ2װ����ȡCu2O��д��ͭ�缫�ĵ缫��Ӧʽ_______��
���𰸡�+75 b B D < > 2Cu+2OH-��2e-=Cu2O+H2O
��������
����1��������֪��Ӧ���Ȼ�ѧ����ʽ�����ݸ�˹���������
��2����ʱŨ����Ϊ![]() =0.6��1������������Ӧ�����ƶ���
=0.6��1������������Ӧ�����ƶ���
����1��A��ƽ��ʱ����ͬһ���ʱ�ʾ�ķ�Ӧ����v��=v�����ò�ͬ���ʱ�ʾ�ķ�Ӧ���ʣ�����֮�ȵ��ڻ�ѧ������֮�ȣ�v(H2)��=2v(CH3OH)����
B������֮�ȵ��ڻ�ѧ������֮�ȣ�2v(CO)=v(H2)��
C��CO��H2��ʼ���ʵ���֮��1��1��ת�������ʵ���֮��1��2����CO��H2ת���ʲ���ȣ�
D������CO��H2�����ʵ���֮�Ȳ��ǰ��ջ�ѧ������֮��ͨ��ģ���ﵽƽ��״̬ʱCO��H2�����ʵ���֮�ȱ��ֲ��䡣
��2�����ȴﵽƽ��ʱ�¶ȸߣ���ͼ��֪�¶���T1������T2ʱ��CO��ת���ʽ��ͣ�˵��ƽ�����淴Ӧ�����ƶ�������K1��K2��
��3��ͭԪ�ػ��ϼ����ߣ�ʧȥ���ӣ�ͭ�缫����������Һ�Լ��ԣ��������缫��ӦʽΪ2Cu��2OH--2e-��Cu2O��H2O��
����1����֪����CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H��+206 kJ/mol
CO(g)+3H2(g) ��H��+206 kJ/mol
��C(s)+H2O(g)��CO(g)+H2(g) ��H��+131 kJ/mol
���ݸ�˹���ɿ�֪�٣��ڼ��õ�CH4(g)��C(s)+2H2(g)�ġ�H��+75kJ/mol��
�ʴ�Ϊ��+75��
��2����ʱŨ����Ϊ![]() =0.6��1������������Ӧ�����ƶ���ѡb��
=0.6��1������������Ӧ�����ƶ���ѡb��
�ʴ�Ϊ��b��
����1���ɷ���ʽCO(g)+2H2(g) ![]() CH3OH(g)��֪��ƽ��ʱ��
CH3OH(g)��֪��ƽ��ʱ��
A��ƽ��ʱ����ͬһ���ʱ�ʾ�ķ�Ӧ����v��=v�����ò�ͬ���ʱ�ʾ�ķ�Ӧ���ʣ�����֮�ȵ��ڻ�ѧ������֮�ȣ�v(H2)��=2v(CH3OH)������A����
B������֮�ȵ��ڻ�ѧ������֮�ȣ�2v(CO)=v(H2)����B��ȷ��
C��CO��H2��ʼ���ʵ���֮��1��1��ת�������ʵ���֮��1��2����CO��H2ת���ʲ���ȣ���C����
D������CO��H2�����ʵ���֮�Ȳ��ǰ��ջ�ѧ������֮��ͨ��ģ���ﵽƽ��״̬ʱCO��H2�����ʵ���֮�ȱ��ֲ�����D��ȷ��
�ʴ�Ϊ��BD��
��2�����ȴﵽƽ��ʱ�¶ȸߣ���ͼ��֪�¶���T1������T2ʱ��CO��ת���ʽ��ͣ�˵��ƽ�����淴Ӧ�����ƶ�������K1��K2��
�ʴ�Ϊ��<��>��
��3��ͭԪ�ػ��ϼ����ߣ�ʧȥ���ӣ�ͭ�缫����������Һ�Լ��ԣ��������缫��ӦʽΪ2Cu��2OH--2e-��Cu2O��H2O��
�ʴ�Ϊ��2Cu+2OH-��2e-=Cu2O+H2O��



