��Ŀ����
8����Ϣ�����ϡ���Դ����Ϊ�¿Ƽ������ġ�����֧�����������й���Ѷ������ǣ�������| A�� | �ڼ�������������Դʱ�������ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ | |
| B�� | �С������յȹ������յ��մɷ������������ܽϴ�̶ȵؽ����ܺģ���Լ��Դ | |
| C�� | ��������Ϣ��ҵ���й㷺Ӧ�ã�������µ���Ҫ�����ǵ��ʹ� | |
| D�� | �ṹ�մ�̼����B4C3�������������������ߣ�����һ���������ǽ������ϣ�����ԭ�Ӿ��� |
���� A�����ܡ�̫���ܡ����ܶ��ǽྻ��Դ��
B���մ������¸��Ȳ��ϣ�
C��ͨ�Ź��µ���Ҫ�ɷ��Ƕ������裻
D���ṹ�մ�̼����B4C3�������������������ߣ�Ӳ�ȸߣ�������ԭ�Ӿ��壮
��� �⣺A�����ܡ�̫���ܡ����ܶ��ǽྻ��Դ����δ����չ������Դ����A��ȷ��
B��Ϊ����߷�������Ч�ʣ�����������ģ������Ҫȡ����ȡ����ȴϵͳ��ʹ���մ����¸��Ȳ��ϣ���B��ȷ��
C����������Ϣ��ҵ���й㷺Ӧ�ã�������µ���Ҫ�����Ƕ������裬��C����
D���ṹ�մ�̼����B4C3�������������������ߣ�Ӳ�ȸߣ�������ԭ�Ӿ��壬��һ���������ǽ������ϣ���D��ȷ����ѡC��
���� ���⿼��������Դ�����ǽ������ϡ��ṹ�մɵ����ʵȣ��ѶȲ���ע��һЩ������֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
4�������ƶϿ��ܺ������ǣ�������
| A�� | Cl-��ClO-��Cl2������ֻ���������ԣ�û�л�ԭ�� | |
| B�� | Cl2����ǿ�����ԣ�����ˮ�е����û����� | |
| C�� | ��ΪSO2���л�ԭ�ԣ���������ˮ���Է�Ӧ����H2SO4��HCl | |
| D�� | Cl2ת��ΪHClOʱһ����Ҫ��������������ʵ�� |
16����1����0.0lmol�������ʢ�Na2O2 ��Na2O ��Na2CO3 ��NaCl�ֱ����100mL����ˮ�У��ָ������£�������Һ��������Ũ�ȵĴ�С˳���Ǣ�=�ڣ��ۣ��ܣ���Һ����仯���Բ��ƣ���
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
��ʵ��1������ƽ�ⳣ��K=2.67��ȡС����λ����ͬ����
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b ��ֵ0��$\frac{a}{b}$��1�������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���10molCO��5molH2O��2molCO2��5molH2�����ʱV����V���������������������=������
��3����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO ��g��+O2��g���T2CO2��g����H=-566.0kJ/mol
��H2O��g���TH2O��l����H=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442.8kJ�Mmol
��4������һNa2CO3��NaHCO3�Ļ������Ʒ��ȡag�û�����ּ��ȣ�����bg��û������Ʒ��Na2CO3������������$\frac{31a-84b}{31a}$��
��5����һ�غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05g��һ�غϽ�����200mLˮ����0.075mol�����������Һ�����������ӵ����ʵ���Ũ����0.75mol/L��������Һ����仯����
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b ��ֵ0��$\frac{a}{b}$��1�������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���10molCO��5molH2O��2molCO2��5molH2�����ʱV����V���������������������=������
��3����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO ��g��+O2��g���T2CO2��g����H=-566.0kJ/mol
��H2O��g���TH2O��l����H=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442.8kJ�Mmol
��4������һNa2CO3��NaHCO3�Ļ������Ʒ��ȡag�û�����ּ��ȣ�����bg��û������Ʒ��Na2CO3������������$\frac{31a-84b}{31a}$��
��5����һ�غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05g��һ�غϽ�����200mLˮ����0.075mol�����������Һ�����������ӵ����ʵ���Ũ����0.75mol/L��������Һ����仯����
3�����й��ڻ�ѧ����ı�ʾ��ȷ���ǣ�������
| A�� | CO2�ĵ���ʽ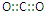 | |
| B�� | CCl4�ı���ģ�ͣ� | |
| C�� | ����18�����ӵ���ԭ�ӵĺ��ط��ţ�${\;}_{17}^{35}$Cl | |
| D�� | ��ԭ�ӵĽṹʾ��ͼ�� |
13�������й���Һ������Ũ�ȵĹ�ϵʽ��һ������ȷ���ǣ�������
| A�� | �����£�pH=7��NH4Cl��NH3•H2O�Ļ����Һ�У�c��Cl-��=c��NH4+����c��OH-��=c��H+�� | |
| B�� | pH=12�İ�ˮ��pH=2���������������Һ�У�c��NH4+����c��Cl-����c��OH-����c��H+�� | |
| C�� | 25��ʱ��0.1 mol•L-1pH=4.5��NaHSO3��Һ�У�c��HSO3-����c��H2SO3����c��SO32-�� | |
| D�� | �����£���Ũ�ȵ�CH3COONa��CH3COOH�����Һ��c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��] |
20����֪�ڱȽ����ǿ��ʱ�����뿼�������棺һ�������������ӵ������������ܼ����������ӵ�����������HCl��ˮ����ǿ�ᣬ�ڱ������о��������HAc��ˮ�������ᣬ��Һ����ȴ��ǿ�ᣬ������Ϊ���������ӵ�������NH3��H2O��HAc�����й�������ǿ����˵����ȷ�ǣ�������
| A�� | ��A�ܼ��У������Դ����ң���B�ܼ��У������Կ���С���� | |
| B�� | HNO3��HClO4�����ᣬ��H2SO4�п����Ǽ� | |
| C�� | �Ƚ�HCl��H2SO4��HClO4��HNO3���Ե�ǿ������ˮ�϶����ܣ��ñ�������ܿ��� | |
| D�� | �Ƚ�HCN��HAc���Ե�ǿ������ˮ�϶����ԣ���Һ���϶�Ҳ���� |
17��������������茶���[��NH4��2SO4•FeSO4•6H2O]���þ����к���Fe2+��NH4+��SO42-���ӵ�ʵ�鷽������ȷ���ǣ�������
| A�� | ȡ������Ʒ���Թܣ���ˮ�ܽ⣬ͨ������Cl2���ټ�KSCN��Һ���۲����� | |
| B�� | ȡ������Ʒ���Թܣ���ˮ�ܽ⣬����NaOH��Һ��¶���ڿ����У��۲����� | |
| C�� | ȡ������Ʒ���Թܣ���ˮ�ܽ⣬����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ��ɫ�ı仯 | |
| D�� | ȡ������Ʒ���Թܣ���ˮ�ܽ⣬����������ټ���BaCl2��Һ�۲����� |
18��ʵ���ҿ��Գ��ڷ��õ��Լ��ǣ�������
| A�� | Na2CO3��Һ | B�� | ������Һ | C�� | ������ | D�� | Cu��OH��2����Һ |