��Ŀ����
��10�֣��±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
�Իش��������⣺
��1�����е�һ��������С��������������������ĸ����ͬ������һ������������������������c��d��e����Ԫ�صĵ�һ�������ɴ�С��˳������������������������������aԪ���γɵ������������ȶ�����ǿ�������������������������ѧʽ����
��2�������������ɵ縺������Ԫ����縺����С��Ԫ����ɵĻ����ﻯѧʽ����������
��3�����л�̬ԭ�ӵ�δ�ɶԵ���������Ԫ��������������������ӵ���Χ�����Ų�ʽ����������
��4��д����a��d��e����Ԫ����ɵ�һ�����ӻ�����Ļ�ѧʽ���������������������ӷ���ʽ��˵����ˮ��Һ�����Ե�ԭ����������������������������������������
| a | | | |||||||||||||||
| b | | | | c | d | e | f | | |||||||||
| g | h | i | j | | k | l | m | ||||||||||
| n | | | | | | | o | | | | | | | | | | |
��1�����е�һ��������С��������������������ĸ����ͬ������һ������������������������c��d��e����Ԫ�صĵ�һ�������ɴ�С��˳������������������������������aԪ���γɵ������������ȶ�����ǿ�������������������������ѧʽ����
��2�������������ɵ縺������Ԫ����縺����С��Ԫ����ɵĻ����ﻯѧʽ����������
��3�����л�̬ԭ�ӵ�δ�ɶԵ���������Ԫ��������������������ӵ���Χ�����Ų�ʽ����������
��4��д����a��d��e����Ԫ����ɵ�һ�����ӻ�����Ļ�ѧʽ���������������������ӷ���ʽ��˵����ˮ��Һ�����Ե�ԭ����������������������������������������
��10�֣������һ��2�֣�����ÿ��1�֣�
��n��m��d��e��c���� ��NaCl��
��o(Fe)��Fe2+��3d6�� ��NH4NO3��NH4++H2O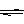 NH3��H2O+H+
NH3��H2O+H+
��n��m��d��e��c���� ��NaCl��
��o(Fe)��Fe2+��3d6�� ��NH4NO3��NH4++H2O
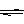 NH3��H2O+H+
NH3��H2O+H+��1��������Խǿ����һ������ԽС�������n�����ء�ϡ������Ԫ���Ѿ��ﵽ�ȶ��ṹ�����һ��������ǽ�����Խǿ����һ������Խ����ԭ�ӵ�2p������Ӵ��ڰ����״̬�����ȶ���ǿ����һ�����ܴ�����Ԫ�صģ���d��e��c���ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ����H2O��NH3��CH4��
��2�����������е縺������Ԫ����縺����С��Ԫ�طֱ����ƺ�Cl���γɵ����ʵĻ�ѧʽΪNaCl��
��3�����л�̬ԭ�ӵ�δ�ɶԵ���������Ԫ������Ԫ�أ����ݹ���ԭ����֪������������ӵ���Χ�����Ų�ʽ��3d6��
��4��a��d��e����Ԫ�طֱ���H��N��O���γɵĻ�������NH4NO3��ˮ�������ԣ�����ʽΪNH4++H2O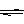 NH3��H2O+H+��
NH3��H2O+H+��
��2�����������е縺������Ԫ����縺����С��Ԫ�طֱ����ƺ�Cl���γɵ����ʵĻ�ѧʽΪNaCl��
��3�����л�̬ԭ�ӵ�δ�ɶԵ���������Ԫ������Ԫ�أ����ݹ���ԭ����֪������������ӵ���Χ�����Ų�ʽ��3d6��
��4��a��d��e����Ԫ�طֱ���H��N��O���γɵĻ�������NH4NO3��ˮ�������ԣ�����ʽΪNH4++H2O
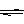 NH3��H2O+H+��
NH3��H2O+H+��
��ϰ��ϵ�д�
�����Ŀ
