��Ŀ����
����Ŀ��ҩ���м���Q��ҽ�ò���PVA�ĺϳ�·����ͼ��
��֪����R-Cl+2NH3��R-NH2+NH4Cl
��R-NO2![]() R-NH2
R-NH2
��-NH2+![]()
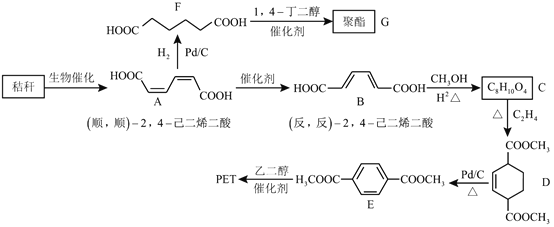
(1)A�ķ���ʽ��________��
(2)B��C��������Ӧ���Լ�a��________(�����ƣ���
(3)C��Dת���Ļ�ѧ����ʽ��________��
(4)E�Ľṹ��ʽ��________��
(5)F���еĹ�������________(�����ƣ������京����ͬ�����ŵ�ͬ���칹�廹��________�֡�
(6)G��X�Ļ�ѧ����ʽ��________��
(7)W�ܷ������۷�Ӧ���γɵĸ߷��ӽṹ��ʽ��________��
(8)P�Ľṹ��ʽ��________��
���𰸡�C6H6 Ũ���ᡢŨ���� ![]() +2NH3
+2NH3![]()
 +NH4Cl
+NH4Cl 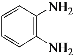 ̼̼˫�������� 4
̼̼˫�������� 4 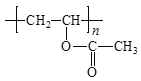 +nH2O
+nH2O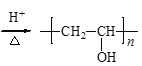 +nCH3COOH
+nCH3COOH 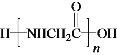
��������
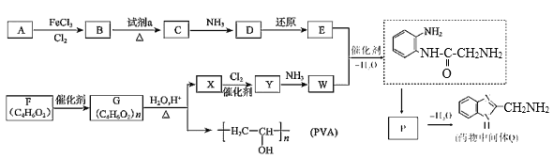
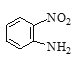
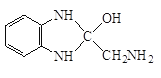
����Q�ṹ��ʽ��A����ʽ֪��AΪ![]() ��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ
��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ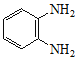 ��DΪ
��DΪ![]() ��CΪ
��CΪ![]() ���Լ�aΪŨ���ᡢŨ���
���Լ�aΪŨ���ᡢŨ���
W�ܷ����ۺϷ�Ӧ�����Q��E�ṹ��ʽ֪��WΪH2NCH2COOH��Y����ȡ����Ӧ����W��X����ȡ����Ӧ����Y��YΪClCH2COOH��XΪCH3COOH��Gˮ������X��PVA����GΪ![]() ��FΪCH3COOCH=CH2��
��FΪCH3COOCH=CH2��
(1)AΪ��������ʽ��C6H6��
(2)B��C��������Ӧ���Լ�a��Ũ���ᡢŨ���
(3) CΪ![]() ��DΪ
��DΪ![]() ��C��DΪȡ����Ӧ���仯ѧ����ʽ��
��C��DΪȡ����Ӧ���仯ѧ����ʽ��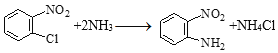 ��
��
(4)E�Ľṹ��ʽ�� ��
��
(5)FΪCH3COOCH=CH2��F���еĹ�������̼̼˫�������������京����ͬ�����ŵ�ͬ���칹�廹��HCOO-C(CH3)=CH2��HCOOCH=CH-CH3��HCOOCH2-CH=CH2��CH2=CH-COOCH3����˹������ֲ�ͬ�ṹ��
(6)GΪ![]() ��FΪCH3COOCH=CH2��G��X�Ļ�ѧ����ʽ�ǣ�
��FΪCH3COOCH=CH2��G��X�Ļ�ѧ����ʽ�ǣ� +nH2O
+nH2O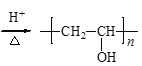 +nCH3COOH��
+nCH3COOH��
(7) WΪH2NCH2COOH��W�����к���-NH2��-COOH������֮���ܷ����ۺϷ�Ӧ���γɵĸ߷��ӽṹ��ʽ��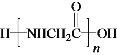 ��
��
(8) 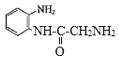 ������-NH2�Ͽ�N-H�����ʻ��Ͽ�C=O˫���������ʻ��ļӳɷ�Ӧ����
������-NH2�Ͽ�N-H�����ʻ��Ͽ�C=O˫���������ʻ��ļӳɷ�Ӧ����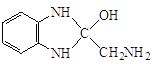 �������ʷ�����ȥ��Ӧ����Q:
�������ʷ�����ȥ��Ӧ����Q: ![]() ������P��
������P�� ��
��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�