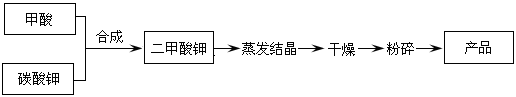
��Ŀ����
WO3�������Ʊ�����Ԫ���������������ȡ��乤ҵ�����������£�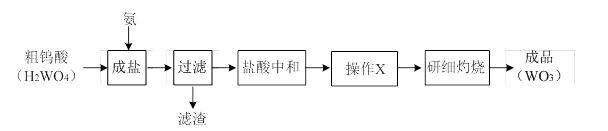
��1������X��Ŀ����Ϊ�˻�ô�����������茶��壬�ò������������������кͺ����Һ ����ȴ�ᾧ�� �����º�ɡ�
��2��ʵ�ʹ�ҵ�����У���������茶��壨������NH4Cl���壩�ɲ����ᴿ��ֱ�����գ���ԭ���� ��
��3����֪��������茶���[x(NH4)2O��yWO3��zH2O]���ȷֽ�Ļ�ѧ����ʽ���£�
x(NH4)2O��yWO3��zH2O��WO3 +NH3��+H2O��(δ��ƽ)��
ijͬѧΪ�ⶨ������茶������ɣ���������ʵ�飺
��ȷ��ȡ16.21g��Ʒ����ϸ���գ�
�ڽ�����������ͨ��װ�м�ʯ�Ҹ���ܣ�������ճƵø��������1.44g��
�۳�����ȴ��Ĺ�������Ϊ13.92g��
ͨ������ȷ����������茶���Ļ�ѧʽ��д��������̣���
��1������Ũ�� ���ˡ�ϴ��
��2������NH4Cl�����շֽ��ȫ��ת��Ϊ����
��3��m(NH3)=16.21g-13.92g-1.44g=0.85g
n(NH4+)=n(NH3)=" 0.85g" ��17g��mol��1=0.05mol
��Ʒ�нᾧˮ��n(H2O)="1.44" g ��18 g��mol��1-0.05mol��2="0.055" mol
n(WO3)=13.92g��232 g��mol��1=0.06mol
x��y��z="0.025" mol��0.06mol��0.055 mol=5��12��11
��������茶���Ļ�ѧʽΪ5(NH4)2O��12WO3��11H2O��
���������������1������Һ����Ũ���������ȴ�ᾧ����������Һ�ֿ����ù��˵ķ�������Ҫϴ�ӳ�ȥ����������ʣ���2��NH4Cl���ȷֽ�ΪHCl��NH3���ӹ����з��룻
��3��������ɵļ���
���ݢڲ����������Ϊˮ����
��Ʒ�нᾧˮ��n(H2O)="1.44" g ��18 g��mol��1-0.05mol��2="0.055" mol
���������غ���������������������NH4+����
m(NH3)=16.21g-13.92g-1.44g=0.85g
n(NH4+)=n(NH3)=" 0.85g" ��17g��mol��1="0.05mol"
���ݢ۲��õ�����ΪWO3��n(WO3)=13.92g��232 g��mol��1=0.06mol
������x��y��z="0.025" mol��0.06mol��0.055 mol=5��12��11
��������茶���Ļ�ѧʽΪ5(NH4)2O��12WO3��11H2O��
���㣺���鹤ҵ����ԭ����������ɼ����й����⡣

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д���֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��ԼΪ mol��L��1��
(2)ijͬѧȡ100 mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)�� mol��L��1��
(3)��ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ���� (�����)��
| A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ������� |
| B������ƿ������ˮϴ����Ӧ��ɺ����������Һ���� |
| C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ�� |
| D����Ҫ����NaClO���������Ϊ143.0 g |

(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������2 000 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ mol��L��1��
������Ũ��������Ϊ mL��
���ǻ��õļ����Ԫ�أ��Ƽ��仯�������������������й㷺��Ӧ�á�
������м��㣺
(1)��������(NaN3)��ײ����ȫ�ֽ�����ƺ͵������ʿ�Ӧ����������ȫ���ҡ�������40.32 L(��״����)������������Ҫ��������________g��
(2)���غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05 g���غϽ�����200 mLˮ����0.075 mol������
�ټ�����Һ�����������ӵ����ʵ���Ũ��(������Һ����仯)��
_____________________________________________________________
�ڼ��㲢ȷ�������غϽ�Ļ�ѧʽ��
_____________________________________________________________
(3)����������Һ�����������ˣ��õ��������Ƶ���Һ�������Һ��ͨ�������̼�������з�Ӧ��
2NaAlO2��3H2O��CO2=2Al(OH)3����Na2CO3
��֪ͨ�������̼336 L(��״����)������24 mol Al(OH)3��15 mol Na2CO3����ͨ����Һ�Ķ�����̼Ϊ112 L(��״����)���������ɵ�Al(OH)3��Na2CO3�����ʵ���֮�ȡ�
_________________________________________________________________
(4)�����£���ȡ��ͬ����������Ʒ����ˮ���������к���pH��7��Ȼ����Һ���ɵ��Ȼ��ƾ��壬���ɹ����в�Ʒ����ʧ��
| | ������������(g) | �Ȼ�������(g) |
| �� | 2.40 | 3.51 |
| �� | 2.32 | 2.34 |
| �� | 3.48 | 3.51 |
����ʵ��٢ڢ������������ƾ��������ʣ���ʵ�����ݿɿ���ͨ�����㣬�����ͱȽ��ϱ�3�����ݣ��������ۡ�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ�������(N2H4)�͵����ᶼ�ǵ�Ԫ�ص���Ҫ�⻯��ڹ�ũҵ�����������������ش����á�
(1)�ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ��������ҪӰ�졣
���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��N2(g)��3H2(g)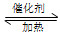 2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
��÷�Ӧ��ƽ�ⳣ���ı���ʽΪ________���ж�K1________K2(�����������������)��
�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����________(����ĸ)��
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2
b��v(N2)����3v(H2)��
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��һ���¶��£���1 L�ܱ������г���1 mol N2��3 mol H2������������Ӧ���������ݻ��㶨��10 min�ﵽƽ��ʱ������������ʵ���Ϊԭ����
 ����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��
����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��(2)�¿����ڻ��ȼ�ϡ���ҩԭ�ϵȡ�
���ڻ���ƽ�����װ����(N2H4)��Һ̬H2O2����֪0.4 molҺ̬N2H4������Һ̬H2O2��Ӧ��������̬N2����̬H2O���ų�256.6 kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ________________________________________________________________________��
��һ����ȼ�ϵ�صĹ���ԭ����ͼ��ʾ���õ�ع���ʱ�����ĵ缫��ӦʽΪ_____________________________________��

�ۼ�����������Һ̬��(N2H4)��ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2���÷�Ӧ�Ļ�ѧ����ʽ_______________________________________��
����������(HNO2)��Ӧ�����ɵ����ᣬ8.6 g��������ȫ�ֽ� �ɷų�6.72 L����(��״����)���������ķ���ʽΪ________��
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
| ���� ����ʽ��HCl ��Է���������36.5,�ܶȣ�1.19 g��cm��3 HCl������������36.5% |
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__ ____mol��L��1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ___ _____mL����Ũ����������ơ�
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(����������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족)��
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ( )
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ ( )
��4���ټ����ͬѧ�ɹ�������0.400 mol��L��1�����ᣬ�����ø������кͺ�0.4 g NaOH��NaOH��Һ�����ͬѧ��ȡ________mL���ᡣ����ȷ��С�����һλ��
�ڼ����ͬѧ�������Ƶ������кͺ�0.4 g NaOH��NaOH��Һ�����ֱȢ����������ƫС������ܵ�ԭ����________��
A��Ũ����ӷ���Ũ�Ȳ���
B��������Һʱ��δϴ���ձ�
C��������Һʱ����������ƿ�̶���
D����ˮʱ�����̶��ߣ��ý�ͷ�ι�����