��Ŀ����
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�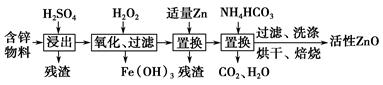
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ_______________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
��(1)49.9(50.0Ҳ����)
(2)��2.2��10��6
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
����

ʵ������Ҫ0.80 mol��L��1 NaOH��Һ475 mL ��0. 40 mol��L��1����500 mL��������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����__________(����)������������Һ�����õ��IJ���������__________(����������)��
(2)����ƿ�����߱��Ĺ�����__________(����)��
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��������Һ |
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��__________0.80 mol��L��1(����ڡ�����С�ڡ����ڡ�����ͬ)������δ����Һ��ȴ�Ͷ����ˣ���������ҺŨ��____________0.80 mol��L��1��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ��������Ϊ________mL(����������һλС��)�����ʵ������10 mL��15 mL��20 mL��50 mL����Ͳ�����ѡ��________mL����Ͳ��
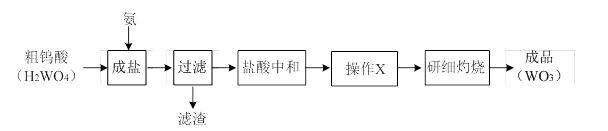

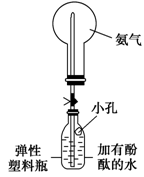
 NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�
NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�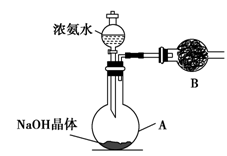
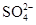 ��= ______mol/L��
��= ______mol/L��