��Ŀ����
14��C60�����ʯ��ʯī�Ľṹģ����ͼ��ʾ��ʯī����ʾ�����е�һ��ṹ����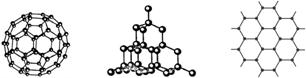
��1��C60�����ʯ��ʯī���ߵĹ�ϵ��ΪB
A��ͬ���칹�� B��ͬ�������� C��ͬϵ�� D��ͬλ��
��2����̬ʱ��C60���ڷ��Ӿ��壨����ӡ���ԭ�ӡ����ӡ�����C60�����к���˫������Ŀ��30��
��3���辧��Ľṹ�����ʯ���ƣ�1mol�辧���к��й�赥������ĿԼ��2NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ����赥��֮�����1����ԭ�ӣ���������Ŀռ���״�ṹ�У��衢��ԭ���γɵ���С������ԭ����Ŀ��6��
��4��ʯī��״�ṹ�У�ƽ��ÿ����������ռ�е�̼ԭ������2��
���� ��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壻
��2�����ݾ��幹�����жϾ������ͣ�
��3�����ݽ��ʯ��С�Ļ�Ϊ��Ԫ���ж϶�������Ŀռ���״�ṹ�У�Si��Oԭ���γɵ���С����Ӧ��6��Siԭ�ӣ�ÿ2��Siԭ��֮����1��Oԭ���ж�Oԭ�ӵ���Ŀ��
��4�����þ�̯�����㣮
��� �⣺��1��ͬ��Ԫ�صIJ�ͬ���ʻ���ͬ�������壬C60�����ʯ��ʯī��̼Ԫ�صIJ�ͬ���ʣ�����ͬ�������壻
�ʴ�Ϊ��B��
��2��C60�й������Ƿ��ӣ��������ڷ��Ӿ��壻
��C60���ӽṹģ�Ϳ��Կ�����ÿ��̼ԭ����������̼ԭ�ӳɼ�����֪ÿ��̼ԭ��Ӧ�γ��ĸ����ۼ����ɴ˿�֪ÿ��̼ԭ��Ӧ��һ��̼̼˫������ÿ��̼̼˫������2��̼ԭ�ӹ��ɣ�����C60�����к�˫����ӦΪ60��$\frac{1}{2}$=30��
�ʴ�Ϊ�����ӣ�30��
��3��C��Si�����ڢ�AԪ�أ����γ�4�����ۼ����ھ������1����ԭ����4����ԭ��ͨ��Si-Siֱ��������ÿ����ԭ�Ӻ���2����������1mol�辧���к��й�赥������ĿԼ��2NA����
���ʯ��С�Ļ�Ϊ��Ԫ������������ṹ�����ʯ�ṹ���ƣ�Si��Oԭ���γɵ���С����Ӧ��6��Siԭ�ӣ��辧��ṹ��ÿ�������Ļ�ѧ��֮�����һ��Oԭ�ӣ���Si��Oԭ���γɵ���С����Oԭ�ӵ���Ŀ��6��
�ʴ�Ϊ��2��6��
��4��ʯī��״�ṹ�У�ÿ��̼ԭ�ӱ������������ι��ã�����ƽ��ÿ����������ռ�е�̼ԭ����=6��$\frac{1}{3}$=2��
�ʴ�Ϊ��2��
���� ���⿼���˾����Ľṹ�������ڿ��龧���ṹ�ķ����ͼ��㣬ע�����þ�̯�����㾧���и���ԭ�Ӹ�������Ŀ�Ѷȴ���ؼ�����ϸ�۲쾧���ṹͼ��

������п��һ������ϡ���ᷴӦʱ��������������ͭ��Һ�ܼӿ췴Ӧ�����Ҳ�Ӱ�������
�ڶƲ������������п������������������������������ʴ
�۵��ʱ��Ӧ�ѶƼ����ڵ��۵�����
��ұ����ʱ�����Ե������̬��AlCl3
�ݸ������泣����ʴ����Fe2O3•nH2O��
| A�� | �ڢ� | B�� | �٢ۢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢܢ� |
| A�� | ���ö�����̼����ȫ�������ϣ����Լ���������̼�Ի�����Ӱ�� | |
| B�� | �ø������������������������˵����ʱ���ԭ�� | |
| C�� | ��ֽ�ˮ��������ֲ��ո����������������Ҵ����漰���������ܵ����� | |
| D�� | �������������Ϊ�����ܸ������ӣ�H+�������ʶ����ᣬ���ܽ������ӵ����ʶ��Ǽ������һ���ۣ�Al��OH��3��NaHCO3��������������� |
| A�� | ��������ˮ��Һ����ͬһ�����ʣ�ֻ��״̬��ͬ | |
| B�� | ��ˮ��Һ�ȶ�����ʹ����IJ�����ɫ | |
| C�� | ������������������ˮ������������Һʱ��ҩƷ������� | |
| D�� | ��ˮ��Һ��dz��ɫ�����д̼�����ζ��˵����ˮ����Cl2���� |
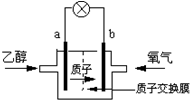 ����ʥ·��˹��ѧ������һ�����͵��Ҵ���أ����û����������ܼ�����200��ʱ���磬�Ҵ���رȼ״����Ч�ʸ߳�32���Ҹ���ȫ������ܷ�ӦΪ��C2H5OH+3O2�T2CO2+3H2O�����ʾ����ͼ������˵������ȷ���ǣ�������
����ʥ·��˹��ѧ������һ�����͵��Ҵ���أ����û����������ܼ�����200��ʱ���磬�Ҵ���رȼ״����Ч�ʸ߳�32���Ҹ���ȫ������ܷ�ӦΪ��C2H5OH+3O2�T2CO2+3H2O�����ʾ����ͼ������˵������ȷ���ǣ�������| A�� | a��Ϊ��صĸ��� | |
| B�� | ��ع���ʱ������b���ص��߾������ٵ�a�� | |
| C�� | ��������ĵ缫��ӦΪ��2H++O2+4e-�TH2O | |
| D�� | ��ع���ʱ1mol�Ҵ�������ʱ����6mol����ת�� |
| A�� |  ��NaOH��������� | B�� | 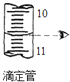 ����Ϊ10.60 | ||
| C�� |  ��Ӧ�ȵIJⶨ | D�� | 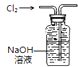 �ռ����� |
| A�� | ��0.1mol/L NaHCO3��Һ�У�c��Na+��+c��H+���Tc��HCO3-��+2c��CO32-�� | |
| B�� | ��0.1mol/L Na2CO3��Һ�У�2c��Na+���Tc��HCO3-��+c��CO32-��+c��H2CO3�� | |
| C�� | ���ʵ���Ũ����ȵ�CH3COOK��CH3COOH��Һ�У���Һ�����ԣ�c��CH3COO-����c��CH3COOH�� | |
| D�� | ���ʵ���Ũ����ȵĢ�NH4HSO4��Һ����NH4HCO3��Һ����NH4Cl��Һ�е�c��NH4+�����٣��ڣ��� |
| A�� | ʵ�ָñ仯��ֻ���������¶� | B�� | ��Һ�ĵ�������һ����ǿ | ||
| C�� | ��Һ��pHһ����С | D�� | ��������ķ����������� |