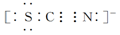��Ŀ����
(14��)��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
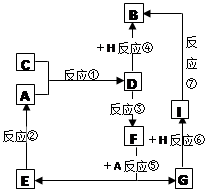
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________________________��
(2)ij��Һ����Mg2����Fe2����Al3����Cu2�����������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2������B��Fe2�� C��Al3�� D��Cu2��
(3)����������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�
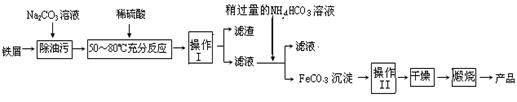
�ش��������⣺
�ٲ������������________���������������________��
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ ��
(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ(5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O)��
a.��ȡ2.850g�̷���FeSO4��7H2O����Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
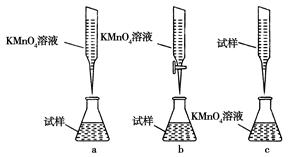
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_____________________________________________��
��ijͬѧ��Ƶ����еζ���ʽ�����������________��(�гֲ�����ȥ)(����ĸ���)
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________________________��
(2)ij��Һ����Mg2����Fe2����Al3����Cu2�����������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2������B��Fe2�� C��Al3�� D��Cu2��
(3)����������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�

�ش��������⣺
�ٲ������������________���������������________��
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ ��
(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ(5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O)��
a.��ȡ2.850g�̷���FeSO4��7H2O����Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_____________________________________________��
��ijͬѧ��Ƶ����еζ���ʽ�����������________��(�гֲ�����ȥ)(����ĸ���)

�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
(14�֣�(1)Fe2����Fe3����H������(2)B��C
(3)�ٹ��ˣ�1�֣���ϴ�ӣ�1�֣�����4FeCO3��O2 2Fe2O3��4CO2
2Fe2O3��4CO2
(4)��250 mL����ƿ����b����0.975(�����Ϊ2�֣�
(3)�ٹ��ˣ�1�֣���ϴ�ӣ�1�֣�����4FeCO3��O2
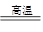 2Fe2O3��4CO2
2Fe2O3��4CO2(4)��250 mL����ƿ����b����0.975(�����Ϊ2�֣�
��1�����ԵĹ���YӦ�����������������������������ᷴӦ����Һ�е������ӷֱ���Fe2����Fe3����H����
��2�����������ǹ����ģ���˵ò���������������������Ϊ�������������ױ���������������������������Ӧ��������������������þ��������ͭ���������պ�����������������þ������ͭ�����������ᷴӦ�����Ȼ������Ȼ�þ���Ȼ�ͭ�����������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������Fe2����Al3��������ѡBC��
��3������������Һ�з�����ķ����ǹ��ˣ�������I�������ǹ��ˡ�̼�����������ں�ɡ�����֮ǰ�������ϴ�ӣ����Բ������������ϴ�ӡ�
�������������Ӽ��ױ������������ڿ���������̼�������ĵĻ�ѧ����ʽ��4FeCO3��O2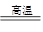 2Fe2O3��4CO2��
2Fe2O3��4CO2��
��4��������������250ml��Һ�����Ի���Ҫ250 mL����ƿ��
����Ʒ��ˮ��Һ�������Եģ�Ӧ������ʽ�ζ��ܡ�ͬ�����������Һ����ǿ�����ԣ�ҲӦ������ʽ�ζ��ܣ����Դ�ѡb��
�۸���5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O��֪��25ml��Һ�к��е��������ӵ����ʵ�����0.01000mol/L��0.02L��5��0.001mol����FeSO4��7H2O������0.001mol��278g/mol��10��2.78g��������Ʒ��FeSO4��7H2O����������Ϊ2.78g��2.850g��0.975����97.5����
��2�����������ǹ����ģ���˵ò���������������������Ϊ�������������ױ���������������������������Ӧ��������������������þ��������ͭ���������պ�����������������þ������ͭ�����������ᷴӦ�����Ȼ������Ȼ�þ���Ȼ�ͭ�����������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������Fe2����Al3��������ѡBC��
��3������������Һ�з�����ķ����ǹ��ˣ�������I�������ǹ��ˡ�̼�����������ں�ɡ�����֮ǰ�������ϴ�ӣ����Բ������������ϴ�ӡ�
�������������Ӽ��ױ������������ڿ���������̼�������ĵĻ�ѧ����ʽ��4FeCO3��O2
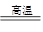 2Fe2O3��4CO2��
2Fe2O3��4CO2����4��������������250ml��Һ�����Ի���Ҫ250 mL����ƿ��
����Ʒ��ˮ��Һ�������Եģ�Ӧ������ʽ�ζ��ܡ�ͬ�����������Һ����ǿ�����ԣ�ҲӦ������ʽ�ζ��ܣ����Դ�ѡb��
�۸���5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O��֪��25ml��Һ�к��е��������ӵ����ʵ�����0.01000mol/L��0.02L��5��0.001mol����FeSO4��7H2O������0.001mol��278g/mol��10��2.78g��������Ʒ��FeSO4��7H2O����������Ϊ2.78g��2.850g��0.975����97.5����

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
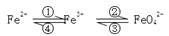

 )��
)��