��Ŀ����
�����������������йط�Ӧ���ʱ䣺
(1)��֪��
Ti(s)��2Cl2(g)===TiCl4(l) ��H����804.2 kJ��mol��1
2Na(s)��Cl2(g)==="2NaCl(s)" ��H����882.0 kJ��mol��1
Na(s)===Na(l) ��H����2.6 kJ��mol��1
��ӦTiCl4(l)��4Na(l)===Ti(s)��4NaCl(s)�Ħ�H�� kJ��mol��1��
(2)��֪���з�Ӧ��ֵ��
��Ӧ�ܵĦ�H4�� kJ��mol��1��
(1)��֪��
Ti(s)��2Cl2(g)===TiCl4(l) ��H����804.2 kJ��mol��1
2Na(s)��Cl2(g)==="2NaCl(s)" ��H����882.0 kJ��mol��1
Na(s)===Na(l) ��H����2.6 kJ��mol��1
��ӦTiCl4(l)��4Na(l)===Ti(s)��4NaCl(s)�Ħ�H�� kJ��mol��1��
(2)��֪���з�Ӧ��ֵ��
| ��� | ��ѧ��Ӧ | ��Ӧ�� |
| �� | Fe2O3(s)��3CO(g)=== 2Fe(s)��3CO2(g) | ��H1����26.7 kJ��mol��1 |
| �� | 3Fe2O3(s)��CO(g)===2Fe3O4(s)��CO2(g) | ��H2����50.8 kJ��mol��1 |
| �� | Fe3O4(s)��CO(g)===3FeO(s)��CO2(g) | ��H3����36.5 kJ��mol��1 |
| �� | FeO(s)��CO(g)===Fe(s)��CO2(g) | ��H4 |
��Ӧ�ܵĦ�H4�� kJ��mol��1��
(1)��970.2 (2)��7.3
(1)����֪��Ӧ�ã�
TiCl4(l)===Ti(s)��2Cl2(g) ��H����804.2 kJ��mol��1��
4Na(s)��2Cl2(g)==="4NaCl(s)" ��H����1764.0 kJ��mol��1��
4Na(s)===4Na(l) ��H����10.4 kJ��mol��1����
���ݸ�˹���ɣ����٣��ڣ��۵ã�
TiCl4(l)��4Na(l)===Ti(s)��4NaCl(s)��H����804.2 kJ��mol��1��1 764.0 kJ��mol��1��10.4 kJ��mol��1����970.2 kJ��mol��1��
(2)���ݸ�˹���ɣ���(�١�3���ڣ��ۡ�2)/6�ã�
FeO(s)��CO(g)===Fe(s)��CO2(g)����H4��(��H1��3����H2����H3��2)/6�֣�7.3 kJ��mol��1��
TiCl4(l)===Ti(s)��2Cl2(g) ��H����804.2 kJ��mol��1��
4Na(s)��2Cl2(g)==="4NaCl(s)" ��H����1764.0 kJ��mol��1��
4Na(s)===4Na(l) ��H����10.4 kJ��mol��1����
���ݸ�˹���ɣ����٣��ڣ��۵ã�
TiCl4(l)��4Na(l)===Ti(s)��4NaCl(s)��H����804.2 kJ��mol��1��1 764.0 kJ��mol��1��10.4 kJ��mol��1����970.2 kJ��mol��1��
(2)���ݸ�˹���ɣ���(�١�3���ڣ��ۡ�2)/6�ã�
FeO(s)��CO(g)===Fe(s)��CO2(g)����H4��(��H1��3����H2����H3��2)/6�֣�7.3 kJ��mol��1��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 FeO(s)��CO(g)
FeO(s)��CO(g)
 CO(g��+ H2(g)��
CO(g��+ H2(g)�� CO (g)+ 3H2(g)
CO (g)+ 3H2(g)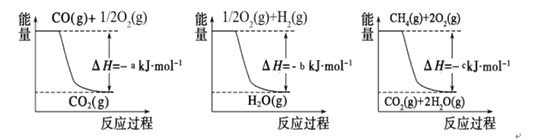
 2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
 2CO2(g)+ N2(g) ��H��0��
2CO2(g)+ N2(g) ��H��0��
 N2O4(g) ��H����56.9 kJ/mol ��
N2O4(g) ��H����56.9 kJ/mol �� 2N2(g) + 3H2O(g)��
2N2(g) + 3H2O(g)��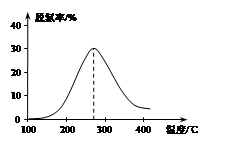

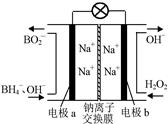
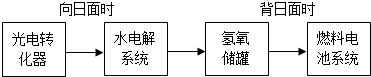
 �������ص�ʹ�ÿ��Լ��ٶԻ�������Ⱦ�������ô������Ϊ��������ҺNaOHΪ���Һ�������س��ʱ������Ӧ ����ŵ�ʱ�������ĵ缫��Ӧ����ʽΪ ��
�������ص�ʹ�ÿ��Լ��ٶԻ�������Ⱦ�������ô������Ϊ��������ҺNaOHΪ���Һ�������س��ʱ������Ӧ ����ŵ�ʱ�������ĵ缫��Ӧ����ʽΪ ��