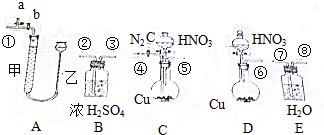
��Ŀ����
1��Ba2+��һ���ؽ������ӣ���һ�������С��������Na2S2O3��K2Cr2O7���Լ��ⶨij������ˮ��Ba2+�����ʵ���Ũ�ȣ���1��������ƿ��ʹ�÷����У����в�������ȷ����b��c������ĸ��ţ���
a��ʹ������ƿǰ�ȼ���Ƿ�©ˮ
b��ʹ������ƿǰ����������ˮ����ϴ��������
c��������Һʱ����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ι���εμ�����ˮ���̶���
d���Ǻ�ƿ������ʳָ��סƿ������һ����סƿ�ף�������ƿ������תҡ��
��2����������250mL0.100mol?L-1�ı�Na2S2O3��Һ������Ҫ�IJ�����������Ͳ��250mL����ƿ���������⣬����Ҫ�ձ�����ͷ�ιܣ�
��3����ȷ��ȡNa2S2O3���������Ϊ4.0g��
��4�������Ʊ�Na2S2O3��Һʱ���������´��������������Һ��Ũ�Ƚ���α仯��
������ϴ��Һת��������ƿʱ����С�����䲿��ϴ��Һ���ᵼ��������Һ��Ũ��ƫ��
���ƫ�ߡ�����ƫ�͡�����Ӱ�족����ͬ����
�����۲�Һ��ʱ��������ƿ�̶��ߣ��ᵼ��������Һ��Ũ��ƫ�ߣ�
��5����ȡ50.00mL��ˮ�������ʵ�����ȣ�����������K2Cr2O7��Һ���õ�BaCrO4������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���������еμ�������Na2S2O3��Һ����Ӧ��ȫʱ������Na2S2O3��Һ36.00mL����֪�йط�Ӧ�����ӷ���ʽΪCr2O72-+6S2O32-+14H+�T2Cr3++3S4O62-+7H2O����ù�����ˮ��Ba2+�����ʵ���Ũ��Ϊ0.024 mol?L-1��
���� ��1����Ϊ����ƿ��һ�־����������ݻ��������¶ȵĸı���ı䣬�������ƿʹ�÷�����ע������ش�����ƿ�Ǿ������ߣ�
��2������������Һ�ܽ⡢���ݵȲ�����ѡ�����������ܽ����ձ��У�����������ƿ���ý�ͷ�ιܵζ����̶ȣ�
��3������200mL 0.100mol/L ��Na2S2O3��Һ������n=cV���������ʵ�����������m=nM�����������������������ƽʹ��ע������õ�������
��4��������ϴ��Һת��������ƿʱ����С�����䲿��ϴ��Һ�����ʼ�С��������ҺŨ�ȼ�С��
�����۲�Һ��ʱ��������ƿ�̶�������ƿ�м���ˮδ�ﵽ�̶ȣ���ҺŨ������
��5����BaCrO4������CrO42-ȫ��ת��ΪCr2O72-��Cr2O72-+6I-+14H+��2Cr3++3I2+7H2O I2+2S2O32-��2I-+S4O62-���ó���ˮ��Ba2+��Na2S2O3�Ĺ�ϵ�����뼴�ɼ��㣮
��� �⣺��1��������ƿ��ʹ�÷����У�
a��ʹ������ƿǰӦ�ü����Ƿ�©ˮ����a��ȷ��
b������ƿ��ˮϴ�������ô�����Һϴ�ӣ������Ӱ��������Һ��Ũ�ȣ���b����ȷ��
c��������Һʱ����������ǹ��壬Ӧ�����ձ����ܽ⣬��ҩƷ��ȫ�ܽ�ָ������£��ٰ���ҺС�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ����ܰѳƺõĹ�����ֽ����������ƿ�У���c����ȷ��
d���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ���d��ȷ��
���bc��
��2������Һ������Ҫ���ձ��У�����ʱ��Ҫ�ý�ͷ�ιܣ��ʴ�Ϊ���ձ�����ͷ�ιܣ�
��3��Na2S2O3�����ʵ���Ϊ0.25L��0.1mol/L=0.025mol��������Ϊ0.025mol��158g/mol=3.95g��
������ƽ��ȷ��Ϊ0.1g��ʹ�ó���Na2S2O3������Ϊ4.0g��
�ʴ�Ϊ��4.0��
��4��������ϴ��Һת��������ƿʱ����С�����䲿��ϴ��Һ�����ʼ�С��������ҺŨ�ȼ�С�����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�����۲�Һ��ʱ��������ƿ�̶�������ƿ�м���ˮδ�ﵽ�̶ȣ���ҺŨ�����ᵼ��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��5����ù�����ˮ��Ba2+�����ʵ���Ϊx��
��BaCrO4������CrO42-ȫ��ת��ΪCr2O72-��Cr2O72-+6I-+14H+��2Cr3++3I2+7H2O��I2+2S2O32-��2I-+S4O62-��
��2Ba2+��2BaCrO4��Cr2O72-��3I2��6S2O32-��
��2 6
��x 36.00mL��10-3L��0.100mol/L
$\frac{2}{x}$=$\frac{6}{36.0��1{0}^{-3}L��0.100mol/L}$��
���x=12.00��10-4mol��
��c��Ba2+��=$\frac{12.00��1{0}^{-4}mol}{50.00��1{0}^{-3}L}$=0.024mol/L��
�ʴ�Ϊ��0.024mol/L��
���� ���⿼���˺������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨��������Ŀ�Ѷ��еȣ�

| A�� | 0.1mol•L-1��NaCl��Һ��Cl-����ĿΪ0.1NA | |
| B�� | 16g O3����������ԭ����ΪNA | |
| C�� | 22.4L H2�к��е���ԭ����һ��Ϊ2NA | |
| D�� | 1mol Cl2������Ľ����Ƴ�ַ�Ӧ��ת�Ƶĵ�����ΪNA |
| A�� | ���Ƿ��ȷ�Ӧ�����Է���Ӧ | |
| B�� | ����������ķ�Ӧ�����Է���Ӧ | |
| C�� | ���Է��Ļ�ѧ��Ӧ��һ���ܷ�����������ȫ | |
| D�� | �жϷ�Ӧ���еķ���Ӧ�ۺϿ�����ϵ���ʱ���ر� |
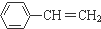 �����������У�����ȷ���ǣ�������
�����������У�����ȷ���ǣ�������| A�� | ����ϩ���Է����Ӿ۷�Ӧ | |
| B�� | ���������ͱ���ϩ���ȼ������CO2�����ʵ������ | |
| C�� | ����ϩ��������1��3�ӳ� | |
| D�� | ����ϩ�����е�ԭ���п��ܶ���ͬһƽ���� |
| A�� | H2S��H2O��HF���ȶ���������ǿ | |
| B�� | RbOH��KOH��Mg��OH��2�ļ������μ��� | |
| C�� | Na+��Mg2+��Al3+������������� | |
| D�� | H2SiO3��H2CO3��H2SO4 ����������ǿ |
| A�� | 0.1mol NH3��������ˮ�У������ǰ����ӷ�������Һ��Nԭ�ӵ���ĿΪ0.1NA | |
| B�� | ��״���£�22.4LNO��CO2�Ļ�������к���Oԭ����Ϊ3NA | |
| C�� | 58.5 g��NaCl�����к���NA���Ȼ��Ʒ��� | |
| D�� | 0.1molN2��������H2��Ӧ��ת�Ƶĵ�������0.6NA |
��1�����飨2������3������ϩ��4������Ȳ ��5��2-��Ȳ ��6�������� ��7���ڶ��ױ� ��8������ϩ��
| A�� | ��3����4����5����8�� | B�� | ��4����5����7����8�� | C�� | ��4����5����8�� | D�� | ��3����4����5����7����8�� |