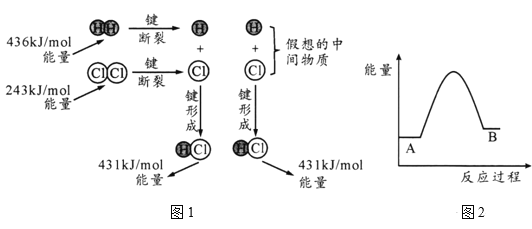
��Ŀ����
����Ŀ����������������ýȾ���������Լ��������ȡ�
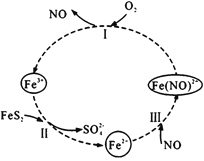
(1)��������������м�ڵ���������ϡ�����Ƶã���ԭ����ΪNO����Ӧ�Ļ�ѧ����ʽΪ________��
(2)ijС��Ϊ̽����������������ȷֽ�������ͼ��ʾװ�ý���ʵ�顣
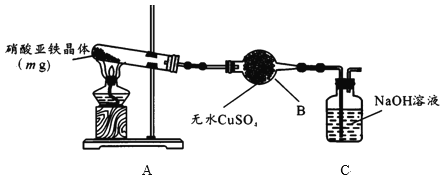
������B��������____��ʵ������ˮCuSO4�������ɴ˿�֪�����������庬��___��
��ʵ���й۲쵽Aװ�õ��Թ����к���ɫ�������ɣ������ȷֽ�����Ϊ��������ʵ�鷽��Ϊ��
ʵ�鲽�� | ���� |
ȡ�������������ϡ���ᣬ������Һ�ֳ����� | �����ܽ�û�ɫ��Һ |
һ�ݵ���____ | ��Һ��ɺ�ɫ |
��һ�ݵ���1��2��K3 [Fe(CN)6]��Һ | ___________ |
A��������������[Fe(NO3)2��xH2O]�ֽ�Ļ�ѧ����ʽΪ______ ��
(3)����̽��mg����������������Ԫ�ص���������
��ȡA���ȷֽ��Ĺ��������ƿ����ϡ�����ܽ⣬���������KI��Һ������2��___��ָʾ����
����a mol/LNa2S2O3����Һ�ζ���ƿ�е���Һ(��֪��I2+2S2O32-=2I-+S4O62-)���ζ����յ�ʱ��ȥbmLNa2S2O3��Һ������������������������������Ϊ______��
���𰸡�3Fe+8HNO3(ϡ)=3Fe(NO3)2+2NO��+4H2O �����(�����θ����) �ᾧˮ l~2��KSCN��Һ ����ɫ�������� 4Fe(NO3)2xH2O![]() 2Fe2O3+8NO2��+O2��+4xH2O ������Һ
2Fe2O3+8NO2��+O2��+4xH2O ������Һ ![]()
��������
(1) ���ڵ���������ϡ���ᣬ��Ӧ��������������NO���ݴ���д��Ӧ�Ļ�ѧ����ʽ��
(2)����ˮCuSO4������˵��������ˮ���ݴ˽�𣻢�ʵ���й۲쵽Aװ�õ��Թ����к���ɫ�������ɣ�������Ϊ����������ͬʱ�������������ݴ���д�ֽ�Ļ�ѧ����ʽ������������������ϡ���ᣬ�õ����������ӵ���Һ������������KSCN���ɫ�� K3 [Fe(CN)6]��Һ�Ǽ����������ӵ��Լ����ݴ˷������
(3)�������Ӿ��������ԣ����������KI��Һ���ܹ����������������ɵⵥ�ʣ��ݴ�ѡ��ָʾ�����ڸ��ݷ����ķ�Ӧ2Fe3++2I-=2Fe2++I2��I2+2S2O32-=2I-+S4O62-�������
(1) ���ڵ���������ϡ���ᣬ��Ӧ��������������NO����Ӧ�Ļ�ѧ����ʽΪ3Fe+8HNO3(ϡ)=3Fe(NO3)2+2NO��+4H2O���ʴ�Ϊ��3Fe+8HNO3(ϡ)=3Fe(NO3)2+2NO��+4H2O��
(2)�ٸ���װ��ͼ������BΪ����ܣ�ʵ������ˮCuSO4������˵��������ˮ���ɴ˿�֪�����������庬�нᾧˮ���ʴ�Ϊ������ܣ��ᾧˮ��
��ʵ���й۲쵽Aװ�õ��Թ����к���ɫ�������ɣ�������Ϊ����������ͬʱ��������������������������[Fe(NO3)2��xH2O]�ֽ�Ļ�ѧ����ʽΪ4Fe(NO3)2xH2O![]() 2Fe2O3+8NO2��+O2��+4xH2O�����ݼ�����������ʵ�鲽�裺����������������ϡ���ᣬ�õ����������ӵ���Һ������������KSCN���ɫ�� K3 [Fe(CN)6]��Һ�Ǽ����������ӵ��Լ�����˵���1��2��K3 [Fe(CN)6]��Һ������ɫ�������ɣ��ʴ�Ϊ��l��2��KSCN��Һ������ɫ�������ɣ�4Fe(NO3)2xH2O
2Fe2O3+8NO2��+O2��+4xH2O�����ݼ�����������ʵ�鲽�裺����������������ϡ���ᣬ�õ����������ӵ���Һ������������KSCN���ɫ�� K3 [Fe(CN)6]��Һ�Ǽ����������ӵ��Լ�����˵���1��2��K3 [Fe(CN)6]��Һ������ɫ�������ɣ��ʴ�Ϊ��l��2��KSCN��Һ������ɫ�������ɣ�4Fe(NO3)2xH2O![]() 2Fe2O3+8NO2��+O2��+4xH2O��
2Fe2O3+8NO2��+O2��+4xH2O��
(3)�������Ӿ��������ԣ����������KI��Һ���ܹ����������������ɵⵥ�ʣ���˿���ѡ�õ�����Һ��ָʾ�����ʴ�Ϊ��������Һ��
�ڷ����ķ�Ӧ��2Fe3++2I-=2Fe2++I2��I2+2S2O32-=2I-+S4O62-���й�ϵʽ2Fe3+��I2��2S2O32-�������������������������������Ϊ![]() ��100%=
��100%=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�����Ŀ���о��������ڴ���a(�����b)�����£�CO2��H2��ͬʱ��������ƽ�з�Ӧ����Ӧ���Ȼ�ѧ����ʽ���£�
�� CO2(g)+ 3H2(g)![]() CH3OH(g)+ H2O(g) ��H1= - 53.7 kJ/mol
CH3OH(g)+ H2O(g) ��H1= - 53.7 kJ/mol
�� CO2(g)+ H2(g)![]() CO(g)+ H2O(g) ��H2= + 41.2 kJ/mol
CO(g)+ H2O(g) ��H2= + 41.2 kJ/mol
ijʵ��С�����CO2��H2��ʼͶ�ϱ�Ϊ1��2.2������ͬѹǿ�£�������ͬ��Ӧʱ���õ�ʵ���������£�
ʵ���� | T(K) | ���� | CO2ת����(%) | �״�ѡ����(%) |
1 | 543 | ����a | 12.3 | 42.3 |
2 | 543 | ����b | 10.9 | 72.7 |
3 | 553 | ����a | 15.3 | 39.1 |
4 | 553 | ����b | 12.0 | 71.6 |
����ע���״�ѡ���ԣ�ת����CO2�����ɼ״��İٷֱȡ�
����˵������ȷ����
A. ��ͬ�¶��£��ڸ�ʱ�̴���b��CO2ת����CH3OH�нϸߵ�ѡ����
B. �����������䣬�����¶ȷ�Ӧ����CO2ת��ΪCH3OHƽ��ת��������
C. �����������䣬����Ӧ��ϵѹǿ��Ӧ����ƽ�ⳣ������
D. ��Ӧ�����������д���a��������b�������������ʾ��ͼ���£�