��Ŀ����
1����������泥�NH2COONH4����һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȣ�ij��ѧ��ȤС��ģ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�2NH3��g��+CO2��g��?NH2COONH4��s����H��0���Ʊ���������淋�װ����ͼ1��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�У���������϶�ʱ��ֹͣ�Ʊ���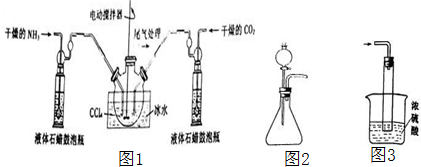
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʣ�
��1������ͼ2װ����ȡ����������ѡ����Լ���Ũ��ˮ����ʯ�һ��������ƹ���ȣ�
��2���������ñ�ˮ��ȴ��ԭ���ǽ����¶ȣ���ƽ�������ƶ�����߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
��3��Һ��ʯ������ƿ��������ͨ���۲����ݣ�����NH3��CO2ͨ��������ӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���ǹ��ˣ���д�������ƣ���
��4��β������װ����ͼ3��ʾ��˫ͨ�����ܵ����ã���ֹ������Ũ��������ã����ն��ఱ������ֹ������ˮ�������뷴Ӧ��ʹ���������ˮ�⣮
��5��ȡ�ֱ��ʵĶ�����̼����淋İ����������Ʒ0.782g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.00g������Ʒ�а����������̼����淋����ʵ���֮��Ϊ4��1��
���� ���������ð����Ͷ�����̼�����Ȼ�̼�л��ܼ���ͨ�������Ƶð�������淋��Ʊ�ʵ�鷽������ƣ������漰��������ʵ�����Ʊ�����Ӧ���¶ȵĿ��ơ�������̼�����Ŀ����Լ���Ӧ�����ķ������ᴿ���Լ���Ʒ���ȷ�����
��1��װ��1�����÷�Һ©������Һ���ܽ���ƿ�еĹ��壬�����ܽ����ʹ��ˮ�ֽ����ɰ�����
��2���˷�Ӧ�ǿ��淴Ӧ������Ӧ�Ƿ��ȷ�Ӧ�����ƽ���ƶ����ۣ�Ϊ��߷�Ӧ���ת���ʣ��轵�´�ƽ��������У�����ҲҪ���Dz���IJ��ȶ��ԣ����¿ɱ������ֽ⣻
��3��Һ��ʯ������ƿ����Ҫ�����ǿ��Ʒ�Ӧ���г̶ȣ������������ٺ�ԭ���������ȣ����ɵİ��������С�������������Ȼ�̼�У������Ʒ��ʵ�鷽�����ù��˵õ���
��4�����ݷ�Ӧ�����еIJ�����������ܰ���Ⱦ�Ե������ŷŵ������У�����������ˮ��������Ҫ�ŵ�����������Խ��Ũ�������ˮ�ԺͲ�Ʒ��ˮ�⿼�ǣ�
��5��̼����淋İ����������Ʒ�У�ʹ̼Ԫ����ȫת��Ϊ̼��ƣ�����̼ԭ���غ�ͻ����������������ߵ����ʵ������ټ�������ǵ����ʵ�����ֵ��
��� �⣺��1����Ũ��ˮ���뵽���������ƻ��������ƣ����ܽ�����з���ʹŨ��ˮ�ֽ����ɰ������ʴ�Ϊ��Ũ��ˮ����ʯ�һ��������ƹ���ȣ�
��2����Ӧ2NH3��g��+CO2��g��?NH2COONH4��s��+Q���Ƿ��ȷ�Ӧ������ƽ��������У��¶����ߣ��������ñ�ˮ��ȴ��߷�Ӧ����ת���ʣ���ֹ�������¶ȹ��߷ֽ⣬�ʴ�Ϊ�������¶ȣ���ƽ�������ƶ�����߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
��3��Һ��ʯ������ƿ�������ǿ��Ʒ�Ӧ���г̶ȣ������������ٺ�ԭ���������ȣ��Ʊ���������淋�װ����ͼ3��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�У������Ʒ��ʵ�鷽�����ù��˵õ����ʴ�Ϊ��ͨ���۲����ݣ�����NH3��CO2ͨ����������ˣ�
��4������������ˮ������Һ�����ײ��ﵹ������˫ͨ�����ܵ������Ƿ�ֹҺ�嵹����Ũ���������ն���İ�����ͬʱ��ֹ������ˮ�������뷴Ӧ��ʹ���������ˮ�⣬�ʴ�Ϊ����ֹ���������ն��ఱ������ֹ������ˮ�������뷴Ӧ��ʹ���������ˮ�⣻
��5��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7820g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000g��̼������ʵ���Ϊ$\frac{1.000g}{100g/mol}$=0.010mol������Ʒ�а�����������ʵ���Ϊx��̼��������ʵ���Ϊy������̼Ԫ���غ�õ���
x+y=0.01 78x+79y=0.7820
���x=0.008mol y=0.002mol
����Ʒ�а����������̼����淋����ʵ���֮��Ϊ$\frac{0.008mol}{0.002mol}$=4��1���ʴ�Ϊ��4��1��
���� ���⿼���������Ʊ�ʵ������Ӧ�ã���Ҫ�ǰ������Ʊ�����������������Ʊ�ʵ��װ�÷����жϣ�ʵ��������������������ʵ����ƣ��йػ����ļ��㣬��Ŀ�Ѷ��еȣ�

��2C��s��+O2��g���T2CO��g����H=-221kJ/mol
��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol
���н�����ȷ���ǣ�������
| A�� | ̼��ȼ����110.5 kJ/mol | |
| B�� | �ٵķ�Ӧ��Ϊ221 kJ/mol | |
| C�� | ϡ������ϡNaOH��Һ��Ӧ���к���Ϊ57.3kJ/mol | |
| D�� | ϡ������ϡNaOH��Һ��Ӧ���к���Ϊ57.3kJ/mol |
| A�� | 32 x | B�� | 32 x mol-1 | C�� | 16 x | D�� | 16 x mol-1 |
| A�� | ����7 | B�� | ����7 | C�� | С��7 | D�� | ��ȷ�� |
| A�� | 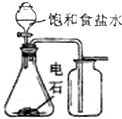 ʵ�����Ʊ����ռ���Ȳ | B�� | 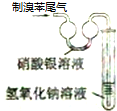 ֤���屽�к���Ԫ�� | ||
| C�� |  ���ȷ�Ӧ | D�� |  ���ͭ��Ũ���ᷴӦ��������� |
 ������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3��
������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3����2��д��ʵ�����Ʊ�A�Ļ�ѧ����ʽCH3COOH+HOCH2CH3$\stackrel{Ũ����}{?}$CH3COOCH2CH3+H2O��
���������
 �dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ�
�dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ�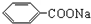 ��]���״�Ϊԭ���Ʊ��������������֪��
��]���״�Ϊԭ���Ʊ��������������֪��| �۵�� | �е�� | ˮ���� | |
| �״� | -97.8 | 64.7 | ���� |
| ������ ��һԪ���ᣩ | 122.4 | 249.3 | ���£�0.17g 100�棺6.8g |
| ��������� | -12.3 | 198 | ���� |

��ش��������⣺
��1���¶Ȣ�ԼΪ64.7�棬������Ϊ��Һ��������Ϊ����
��2���ڢڲ����ȵ�Ŀ�������������ļ״���
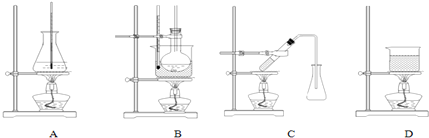
��3��ѡ����ʵ��Ʊ������������װ�ã�B��
��4������������ж���ͬ���칹�壬д��������������������һ��ͬ���칹��Ľṹ��ʽ
 ��
����Ϊ���㻯���� �ں���ȩ�� ���������Na��Ӧ��
��֪��
 ��
����Ӧ�����������������������
| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�����������������Ӧ�
���Թ�C���ڱ�ˮԡ�е�Ŀ���Ƿ�ֹ����ϩ����Ӧ��ӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣨���ϻ��£�����Һ����C������ţ�ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ

���ٽ�����ϩ��ͼ��װ��������ȴˮ��f������ĸ���ڽ��룻����ʱҪ������ʯ�ҵ�Ŀ�ij�ȥˮ�֣�
��ͼ������װ���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����cd��
a������ʱ��70�濪ʼ�ռ���Ʒ��
b��������ʵ���������ˣ�
c���Ʊ���Ʒʱ���������Ʒһ��������
d���ǿ��淴Ӧ����Ӧ�ﲻ��ȫ��ת��
��3�����ֻ���ϩ��Ʒ�ʹ�Ʒ���Ƿ��з�Ӧ��ķ�����ȡ������ƷͶ��һС������ƣ��۲��Ƿ������ݲ�������ⶨ�е��Ƿ�Ϊ83�棩��
