��Ŀ����
17�������軯�أ�K4Fe��CN��6����Ѫ�Σ���ʵ���ҡ���ơ�ʳƷ���Ӽ���ҽѧ�Ͽ�����ұ���裨Tl���ж����������ʱ�����ڻ滭���й㷺��;��һ���ú�NaCN��ˮ�ϳɻ�Ѫ�ε���Ҫ����������ͼ1��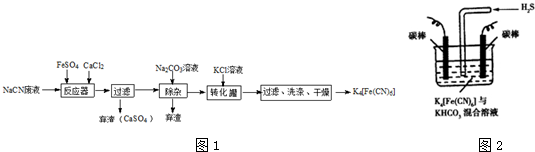
��1��NaCN�ĵ���ʽΪ
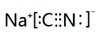 ��
����2������ƽ���ƶ�ԭ��ʵ���ҳ���K4Fe��CN��6��Һ����Fe3+������������KFe[Fe��CN��6]�����ɵ����ֿ���������Tl2SO4�ж�����д��������Ӧ�����ӷ���ʽΪK++[Fe��CN��6]4-+Fe3+=KFe[Fe��CN��6]����
��3������ͼ�м���̼������Һ��ҪĿ���dz�ȥ���е�Ca2+��
��4����ͬ�¶����ܽ�ȣ�Na4[Fe��CN��6]��K4[Fe��CN��6]��ѡ�����������=��������������
��5�����ʻ���[Fe��CO��5]�൱���ã�����H2�������ʻ�����[H2Fe��CO��4]�����ʻ�������NaOH��Ӧ���ɵ����ʻ��������ˮ��Һ�ʼ��ԣ� ���ʻ�������ˮ��Һ�еĵ��뷽��ʽΪH2Fe��CO��4?HFe��CO��4-+H+��HFe��CO��4-?Fe��CO��42-+H+��
��6����ҵ�ϳ�������ͼ2��ʾ�ĵ��װ�ã��������Ļ����ォ��̬�������е�����ת��Ϊ�����õ���ͨ���⣬Ȼ��ͨ��H2Sʱ������Ӧ�����ӷ���ʽΪ2[Fe��CN��6]3-+2CO32-+H2S�T2[Fe��CN��6]4-+2HCO3+S���� ����������������Һ��pH�����������С�����䡱����
��7�������£�����ȱλ����пZnFe2Oy��������NOx��Ⱦ��ʹNOxת��ΪN2��ͬʱZnFe2Oyת��Ϊ ZnFe2O4����2mol ZnFe2Oy������NO2��Ӧ������0.5mol N2����y=3��
���� ʵ��Ŀ���Ǻϳɻ�Ѫ�Σ������̿�֪��NaCN��Һ�м��������������Ȼ��ƣ�����6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��Ȼ�����̼������Һ�ɳ�ȥ������Ca2+�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6��˵��K4[Fe��CN��6�ܽ�Ƚ�С���Դ˽����⣮
��1��NaCNΪ���ӻ�������ӻ����������Ӵ�������á�[]����
��2��K4Fe��CN��6��Fe3+��Ӧ��������������KFe[Fe��CN��6]�ͼ����ӣ�
��3����Ӧ���м����Ȼ��ƣ���Ҫ����̼���Ƴ�ȥ��
��4�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6��˵��K4[Fe��CN��6�ܽ�Ƚ�С��
��5���⻯�ʻ���Ϊ��Ԫ���ᣬ����Һ�з��������룻
��6�����ʱ��������ʧ���ӵ�������Ӧ����[Fe��CN��6]4-ת��ΪFe��CN��6]3-�����ϼ����ߣ�������ӦʽΪ2HCO3-+2 e-�TH2��+2CO32-���ݴ��жϣ�
��7������������ԭ��Ӧ�ĵ����غ����ZnFe2Oy��FeԪ�ػ��ϼۣ��ٸ��ݻ������е�Ԫ�ػ��ϼ۴���Ϊ0����y��ֵ��
��� �⣺ʵ��Ŀ���Ǻϳɻ�Ѫ�Σ������̿�֪��NaCN��Һ�м��������������Ȼ��ƣ�����6NaCN+FeSO4+CaCl2=Na4[Fe��CN��6+CaSO4��+2NaCl��Ȼ�����̼������Һ�ɳ�ȥ������Ca2+�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6��˵��K4[Fe��CN��6�ܽ�Ƚ�С��
��1��NaCNΪ���ӻ��������ʽΪ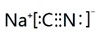 ���ʴ�Ϊ��
���ʴ�Ϊ��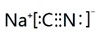 ��
��
��2��K4Fe��CN��6��Fe3+��Ӧ��������������KFe[Fe��CN��6]�ͼ����ӣ����ӷ���ʽΪ��K++[Fe��CN��6]4-+Fe3+=KFe[Fe��CN��6]����
�ʴ�Ϊ��K++[Fe��CN��6]4-+Fe3+=KFe[Fe��CN��6]����
��3����Ӧ���м����Ȼ��ƣ�����̼������Һ�ɳ�ȥ������Ca2+��
�ʴ�Ϊ����ȥ���е�Ca2+��
��4�������˺�����Һ�м���KCl��ת������K4[Fe��CN��6��˵��K4[Fe��CN��6�ܽ�Ƚ�С���ʴ�Ϊ������
��5���⻯�ʻ���Ϊ��Ԫ���ᣬ����Һ�з��������룬����뷽��ʽΪ��H2Fe��CO��4?HFe��CO��4-+H+��HFe��CO��4-?Fe��CO��42-+H+��
�ʴ�Ϊ��H2Fe��CO��4?HFe��CO��4-+H+��HFe��CO��4-?Fe��CO��42-+H+��
��6�����ʱ������ӦʽΪ2HCO3-+2 e-�TH2��+2CO32-������CO32-��ˮ��̶ȴ���HCO3-�����Լ�����ǿ����pH���
�ʴ�Ϊ�����
��7��2mol ZnFe2Oy������NO2������0.5mol N2����ZnFe2Oy����ԭΪZnFe2O4����ZnFe2Oy����Ԫ�صĻ��ϼ�Ϊa�����ݵ���ת���غ㣬��֪2mol��2����3-a��=0.5mol��2��4�����a=2�����ϼ۴�����Ϊ0����2+2��2=2y�����y=3��
�ʴ�Ϊ��3��
���� ���⿼�����ʵ��Ʊ�ʵ�鷽������ƣ�������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬Ϊ�߿��������ͣ�ע���������ͼ����ʵ���ԭ���Ͳ����������Ѷ��еȣ�ע�����������ԭ��Ӧ�Ļ��ϼ۵�����������ȼ��㣮

| A�� | ���ࡢ��֬�͵������ǻ���Ӫ�����ʣ����Ƕ�����Ȼ�߷��ӻ����� | |
| B�� | ������ˮ������ղ����Ƕ��� | |
| C�� | �Ʊ���Ĺ����漰��������Ӧ | |
| D�� | ������Һ�м���Ũ��Na2SO4��CuSO4��Һ����ʹ�����������������ᴿ |
| A�� | Ba��OH��2•8H2O������NH4Cl����ķ�Ӧ��һ���ų������ķ�Ӧ | |
| B�� | ˮ�ֽ��������������ʱ�ų����� | |
| C�� | ��ͬ״���£�2SO2+O2?2SO3��һ�����ȷ�Ӧ����2SO3?2SO2+O2��һ�����ȷ�Ӧ | |
| D�� | 73 g�Ȼ��Ⱦ��е���������2 g������71 g�������е������� |
| A�� | ƽ�ⲻ�����ƶ� | B�� | ƽ�������淴Ӧ�����ƶ� | ||
| C�� | ���淴Ӧ���ʶ����� | D�� | NH3������������С |
| A�� | ���ȴ��飨��Ʒ���������������ȶ���ȼ�գ��ɹ㷺��������� | |
| B�� | ��֬����������ˮ�����ɸ�֬�����κ��ͣ��ڹ�ҵ������������� | |
| C�� | �Ҵ��ӷ��������������л����������ܼ� | |
| D�� | ��ϩ�ܱ�KMnO4����������Ϊˮ������� |

