��Ŀ����
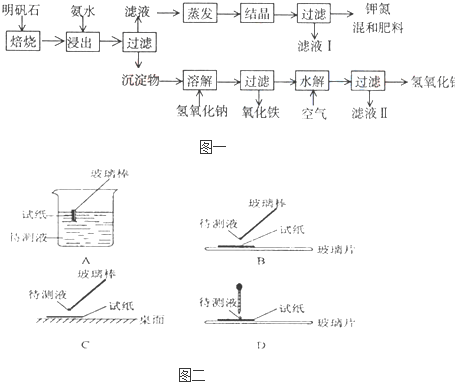
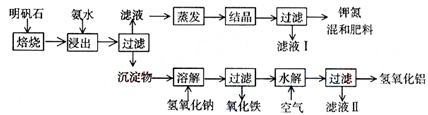
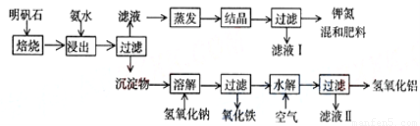
������ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ������������ �����������������ʣ�����ʵ�鲽����ͼһ��ʾ��
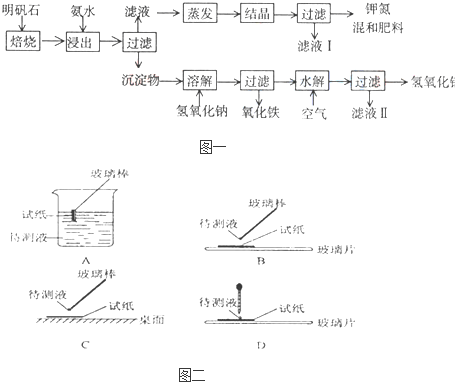
����ͼһʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ ������250.28g����______mL���ù��Ϊ______mL��Ͳ��ȡ��
��2����ˮ������õ���Һ�����ϵ�����ˣ���Һ�г�K+��SO42-�⣬���д�����NH4+������NH4+�ķ�����______��
��3��д�����������������ʵĻ�ѧʽ______��
��4����ҺI�ijɷ���ˮ��______��
��5��Ϊ�ⶨ��Ϸ���K2SO4����NH4��2SO4�мصĺ������������в��裺
�ٳ�ȡ�ص�������������ˮ����������______��Һ��������ɫ������
��______��______��______��������дʵ��������ƣ���
����ȴ�����أ�
��6��������Ϊmg�����������ʵ���Ϊnmol����������K2SO4�����ʵ���Ϊ��______mol���ú�m��n�Ĵ���ʽ��ʾ����
��ij��ɫ����Һ���ܺ����������ӣ�K+��Al3+��Fe3+��Ba2+��NO3-��SO42-��HCO3-��Cl-�ȣ�ȡ����Һ��������ʵ�飺
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬��������������������Ϊ���ء�ɫ��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ��
��ش��������⣺
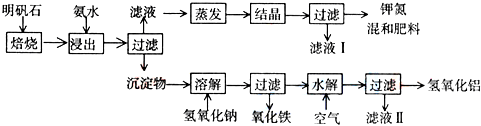
�� l ����ʵ�� ���У�ͼ����ʾ�IJ�������ȷ����______������ţ�
��2����������ʵ���ж�ԭ��Һ�п϶����ڵ�������______���϶������ڵ�������______��
��3��д����ڢ�����ʵ���йص����ӷ���ʽ��
��______��
��______��
�⣺��1������ϡ��ǰ�����ʵ���������ã�0.5L��39.20g/L=V��250.28g/L�����V=0.78L=78mL��Ϊ����������ѡ��Ȱ�ˮ�����һЩ����Ͳ���ɣ���ѡ��100mL��Ͳ��
�ʴ�Ϊ��78��100��
��2��ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ������˵���а����ų�������NH4+���ӣ�
�ʴ�Ϊ��ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ������
��3������Ŀ��Ϣ��֪������ʯ����ɺ��������ƣ�����ʯ�к���Al2O3��Fe2O3�����Գ��������ɵ�Al��OH��3����δ�ܽ�� Al2O3��Fe2O3��
�ʴ�Ϊ��Al��OH��3��Al2O3��Fe2O3��
��4������ʯ����ɺ��������ƣ����ݹ�������ת����ϵ��֪����ҺI�к���K2SO4�ͷ�Ӧ���ɣ�NH4��2SO4��
�ʴ�Ϊ��K2SO4����NH4��2SO4��
��5����Ϸ����к���K2SO4����NH4��2SO4��Ҫ������ɫ�������������ҺΪBaCl2��Ba��NO3��2��Һ��Ȼ����в������Ƚ����Һ���ˣ�Ȼ��ϴ�ӳ������������ȴ����أ�
�ʴ�Ϊ��BaCl2 ��Ba��NO3��2�����ˡ�ϴ�ӡ����
��6������Ϊmg����174n��K2SO4��+132n[��NH4��2SO4]=m�����������ʵ���Ϊnmol����BaSO4�����ʵ���Ϊnmol������������غ��У�n��K2SO4��+n[��NH4��2SO4]=n��������ã�n��K2SO4��=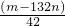 mol��
mol��
�ʴ�Ϊ��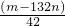 ��
��
��1��A����ֽ�������Һ�У�����Ⱦ�Լ�����A����
B���ò�����պȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飬��B��ȷ��
C����ֽ���ܷ��������ϣ����Է��ڲ���Ƭ���ΰ��ϣ���C����
D��Ӧ�õ���ȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飬��D��ȷ��
�ʴ�Ϊ��BD��
��2����Һ��ɫ����һ��������Fe3+��
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��˵����Һ�����ԣ���һ��������HCO3-��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��������ΪNO��˵����Һ�д���NO3-��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��˵������Al3+���ӣ�
��ȡ��Һ�����������Ȼ�����Һ������ɫ�������ó���Ϊ���ᱵ������˵������SO42-����һ�����Ậ��Ba2+��
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ������֤���Ƿ���Cl-���ӣ�����м����Ȼ�����
�ʴ�Ϊ��һ����Al3+��NO3-��SO42-��һ��û��Fe3+��Ba2+��HCO3-��
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壬��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3����Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
��������1������ϡ�Ͷ��ɼ�����ҪŨ��ˮ�����������ҪŨ��ˮ�����ѡ����Ͳ�Ĺ��Ϊ����������ѡ��Ȱ�ˮ�����һЩ����Ͳ���ɣ�
��2����笠�������ǿ�Ӧ��ת��Ϊ������������ʹʪ��ĺ�ɫʯ����ֽ������
��3������Ŀ��Ϣ��֪������ʯ����Al2O3��Fe2O3��
��4������ʯ����ɺ��������ƣ����ݹ�������ת����ϵ��֪����ҺI�к���K2SO4�ͷ�Ӧ���ɣ�NH4��2SO4��
��5����Ϸ����к���K2SO4����NH4��2SO4��Ҫ������ɫ�������������ҺΪBaCl2��Ba��NO3��2��Һ��Ȼ����в������Ƚ����Һ���ˣ�Ȼ��ϴ�ӳ������������ȴ����أ�
��6������Ϊmg����174n��K2SO4��+132n[��NH4��2SO4]=m�����������ʵ���Ϊnmol����BaSO4�����ʵ���Ϊnmol������������غ��У�n��K2SO4��+n[��NH4��2SO4]=n���ݴ˼��㣮
��1����ֽ������Һ�������ʱ��Ӧ�ò�������ȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飮
��2�����ݷ�Ӧ�������ж����Ӵ��ڵĿ����ԣ�
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壮
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3��д���ӷ���ʽ��
���������⿼��ѧ���Թ������̵����⡢���ӵļ����빲�桢���ʷ����ᴿ�Ȼ������������������뻯ѧ�����ѧ����ȣ���Ŀ�Ѷ��еȣ�����ע�ⳣ�����Ӽ���ķ����Ļ��ۣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������
�ʴ�Ϊ��78��100��
��2��ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ������˵���а����ų�������NH4+���ӣ�
�ʴ�Ϊ��ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ������
��3������Ŀ��Ϣ��֪������ʯ����ɺ��������ƣ�����ʯ�к���Al2O3��Fe2O3�����Գ��������ɵ�Al��OH��3����δ�ܽ�� Al2O3��Fe2O3��
�ʴ�Ϊ��Al��OH��3��Al2O3��Fe2O3��
��4������ʯ����ɺ��������ƣ����ݹ�������ת����ϵ��֪����ҺI�к���K2SO4�ͷ�Ӧ���ɣ�NH4��2SO4��
�ʴ�Ϊ��K2SO4����NH4��2SO4��
��5����Ϸ����к���K2SO4����NH4��2SO4��Ҫ������ɫ�������������ҺΪBaCl2��Ba��NO3��2��Һ��Ȼ����в������Ƚ����Һ���ˣ�Ȼ��ϴ�ӳ������������ȴ����أ�
�ʴ�Ϊ��BaCl2 ��Ba��NO3��2�����ˡ�ϴ�ӡ����
��6������Ϊmg����174n��K2SO4��+132n[��NH4��2SO4]=m�����������ʵ���Ϊnmol����BaSO4�����ʵ���Ϊnmol������������غ��У�n��K2SO4��+n[��NH4��2SO4]=n��������ã�n��K2SO4��=
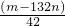 mol��
mol���ʴ�Ϊ��
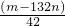 ��
����1��A����ֽ�������Һ�У�����Ⱦ�Լ�����A����
B���ò�����պȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飬��B��ȷ��
C����ֽ���ܷ��������ϣ����Է��ڲ���Ƭ���ΰ��ϣ���C����
D��Ӧ�õ���ȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飬��D��ȷ��
�ʴ�Ϊ��BD��
��2����Һ��ɫ����һ��������Fe3+��
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��˵����Һ�����ԣ���һ��������HCO3-��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ��������ΪNO��˵����Һ�д���NO3-��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��˵������Al3+���ӣ�
��ȡ��Һ�����������Ȼ�����Һ������ɫ�������ó���Ϊ���ᱵ������˵������SO42-����һ�����Ậ��Ba2+��
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ������֤���Ƿ���Cl-���ӣ�����м����Ȼ�����
�ʴ�Ϊ��һ����Al3+��NO3-��SO42-��һ��û��Fe3+��Ba2+��HCO3-��
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壬��Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3����Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
��������1������ϡ�Ͷ��ɼ�����ҪŨ��ˮ�����������ҪŨ��ˮ�����ѡ����Ͳ�Ĺ��Ϊ����������ѡ��Ȱ�ˮ�����һЩ����Ͳ���ɣ�
��2����笠�������ǿ�Ӧ��ת��Ϊ������������ʹʪ��ĺ�ɫʯ����ֽ������
��3������Ŀ��Ϣ��֪������ʯ����Al2O3��Fe2O3��
��4������ʯ����ɺ��������ƣ����ݹ�������ת����ϵ��֪����ҺI�к���K2SO4�ͷ�Ӧ���ɣ�NH4��2SO4��
��5����Ϸ����к���K2SO4����NH4��2SO4��Ҫ������ɫ�������������ҺΪBaCl2��Ba��NO3��2��Һ��Ȼ����в������Ƚ����Һ���ˣ�Ȼ��ϴ�ӳ������������ȴ����أ�
��6������Ϊmg����174n��K2SO4��+132n[��NH4��2SO4]=m�����������ʵ���Ϊnmol����BaSO4�����ʵ���Ϊnmol������������غ��У�n��K2SO4��+n[��NH4��2SO4]=n���ݴ˼��㣮
��1����ֽ������Һ�������ʱ��Ӧ�ò�������ȡ������Һ���ڷ��ڲ���Ƭ����ֽ�ϼ��飮
��2�����ݷ�Ӧ�������ж����Ӵ��ڵĿ����ԣ�
��3���ڷ�ӦΪ����ͭ����������������������ӷ���������ԭ��Ӧ����ͭ���Ӻ�һ���������壮
�۸���Al��OH��3������������ں�Al3+��Һ�м��백ˮֻ����Al��OH��3��д���ӷ���ʽ��
���������⿼��ѧ���Թ������̵����⡢���ӵļ����빲�桢���ʷ����ᴿ�Ȼ������������������뻯ѧ�����ѧ����ȣ���Ŀ�Ѷ��еȣ�����ע�ⳣ�����Ӽ���ķ����Ļ��ۣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�
�����Ŀ