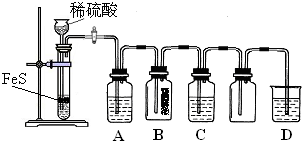
��Ŀ����
�������������ʵ�����Ʒ����������������ա�(1)�������շ�������ȡ�������壬����ȡ�����ԭ�Ͽ�ѡ��____________________��
a.ϡ������������
b.ϡ������������
c.ϡ����������
d.ϡ������������
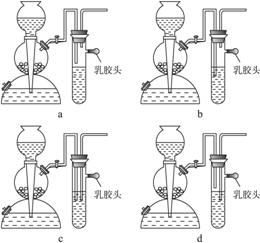
(2)��Ҫ��װһ���Կ�����������������ʵ�װ�ã�������ͼ��ѡ����ʵ�������____________________ (����)��
(3)����ͼ����һ�����������ļ���ƿ�м�������Ʒ��ϡ��Һ����ȼ�������塣�ڻ����Զ�Ϩ���ֹͣͨ�����壬ƿ�ڿɹ۲쵽��������____________________��
(4)����������ƿ�м���ͨ���������壬��������Ӧ�Ļ�ѧ����ʽΪ____________________����Ӧ�����У���Һ��pH____________________ (������С�����䡱)��
(5)��ȼ����������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���____________________��
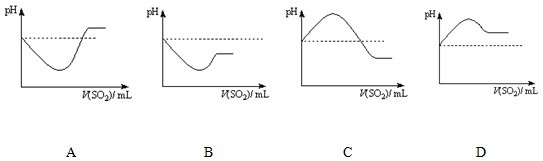
(6)��֪���������ڿ����е��������Ϊ4.3%��45.5%ʱ�ᷢ����ը�������������ڿ����е��������Ϊ30%ʱ���䱬ը������____________________��
��1��a d
(2)�ڢۢ�
(3)Ʒ����Һ��ɫ ƿ���е���ɫ��ĩ����ɫ��СҺ��
��4��SO2+2H2S![]() 3S��+2H2O ���
3S��+2H2O ���
��5��b
(6)
����:��1���������շ�������ȡ���壬��ѡ�Լ�ӦΪ��״�����Һ�壬��a��d��ȷ���������ǿ�����ԣ�����������Ӧ������H2S��b�����
��3����ʼ�������㣬H2Sȼ������SO2��H2O�������������㣬ȼ�ղ���ΪS��H2O��������Ӧ����Ҫ��SO2��S��H2O�������濼�ǡ�
��5��a�����ӵ�װ�����κ����á�bװ�ÿɽ����շ������ڵ�H2S���ȼ��H2S�ָ������ܷ�ֹ��ը��c��dװ�������ɵ�H2S���ų����ʴ���
��6���������ڿ����е��������Ϊ30%ʱ��O2�ڻ�������е��������Ϊ![]() ��O2���㣬������Ӧ2H2S��O2
��O2���㣬������Ӧ2H2S��O2![]() 2S��2H2O��
2S��2H2O��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�