��Ŀ����
����A��B��C��D��E�������ʣ�����������ת����ϵ������EΪ��ɫ��ĩ��

��������ͼ��ʾ��ʵ��װ�ý���C��ˮ�ķ�Ӧ���ش������й�����
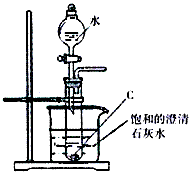
��1������Һ©���е�ˮ�����Թܣ�Ȼ��
���ڵ��ܿڴ������ʵ��������۲�����
�ڹ۲��ձ��е�����Ϊ��__________________________���������۲�����ɵõ�������������_______________________________��
��д���Թ��з�Ӧ�Ļ�ѧ����ʽ____________________________��
��2����֪ˮ��C��Ӧ����D����һ�ֻ�����F����ͨ��״���£�����F��ϡ��Һ�뺬����Ϊ1mol��ϡ������ȫ��Ӧʱ�ų�akJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ___________________________��
���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ��������ͼ��ʾʵ��װ��
���ڵ��ܿڴ������ʵ��������۲�����
�ڹ۲��ձ��е�����Ϊ��__________________________���������۲�����ɵõ�������������_______________________________��
��д���Թ��з�Ӧ�Ļ�ѧ����ʽ____________________________��
��2����֪ˮ��C��Ӧ����D����һ�ֻ�����F����ͨ��״���£�����F��ϡ��Һ�뺬����Ϊ1mol��ϡ������ȫ��Ӧʱ�ų�akJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ___________________________��
���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ��������ͼ��ʾʵ��װ��
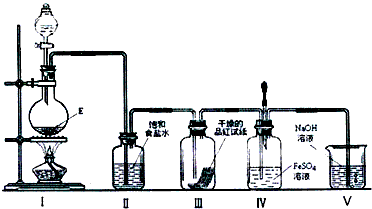
��3����д����ʵ�����Ʊ���������X�����ӷ�Ӧ����ʽ_________________________��
��4��ʵ������У��۲쵽װ�â���Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���_____________________________________��
��5��ʵ�������װ�â��пɹ۲쵽������____________________________�����μ����ν�ͷ�ι��е��Լ����۲쵽��Һ�ʺ�ɫ����ͷ�ι��е��Լ�Ϊ��_______________________________��
��4��ʵ������У��۲쵽װ�â���Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���_____________________________________��
��5��ʵ�������װ�â��пɹ۲쵽������____________________________�����μ����ν�ͷ�ι��е��Լ����۲쵽��Һ�ʺ�ɫ����ͷ�ι��е��Լ�Ϊ��_______________________________��
��1���ڳ��ֻ��ǣ�������ɫ������������Ӧ���ȣ�����������
��2Na2O2+2H2O==4NaOH+O2��
��2��NaOH��aq��+HCl��aq��==NaCl��aq��+H2O��l������H��-akJ/mol ����H+��aq��+ OH-��aq��==H2O��l������H��-akJ/mol��
��3��MnO2+4H++2Cl- Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
��4����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ
��5����Һ��dz��ɫ��Ϊ��ɫ��KSCN�������𰸾��÷֣�
��2Na2O2+2H2O==4NaOH+O2��
��2��NaOH��aq��+HCl��aq��==NaCl��aq��+H2O��l������H��-akJ/mol ����H+��aq��+ OH-��aq��==H2O��l������H��-akJ/mol��
��3��MnO2+4H++2Cl-
 Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O��4����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ
��5����Һ��dz��ɫ��Ϊ��ɫ��KSCN�������𰸾��÷֣�

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ
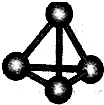 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






