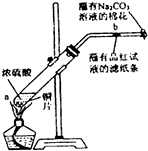
题目内容
2.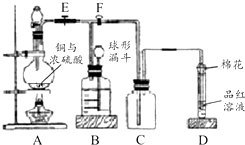 某课外兴趣小组为探究铜跟浓硫酸反应情况,用如图所示装置进行实验.
某课外兴趣小组为探究铜跟浓硫酸反应情况,用如图所示装置进行实验.已知:①SO2难溶于饱和亚硫酸氢钠溶液;②SO2能与酸性高锰酸钾溶液发生氧化还原反应使之褪色(化学方程式为5SO2+2KMnO4+2H2O═K2SO4+2MnSO4+2H2SO4).
回答下列问题(注:E为止水夹,F为旋塞):
(1)检查A装置的气密性的方法关闭止水夹E和分液漏斗的旋塞,向分液漏斗中加水,然后打开分液漏斗旋塞,若漏掉中的水滴入少量后停止,证明气密性良好,若水一直滴下,则气密性不好.
(2)装置A中反应的化学方程式为Cu+2H2SO4(浓)△_△––––––CuSO4+SO2↑+2H2O.
(3)装置D中试管口放置的棉花应蘸有NaOH溶液,其作用是吸收SO2气体,防止污染.
(4)装置B具有贮存气体的作用.当D处有明显的现象后,关闭旋塞F并移去酒精灯,由于余热的作用,A处仍有气体产生,此时B中的现象是集气瓶内液面下降,长颈漏斗内液面上升,B中应放置的液体是b(填字母).
a.水 b.饱和NaHSO3溶液 c.酸性KMnO4溶液 d.NaOH溶液
(5)该小组学生做了如下实验:取一定质量的铜片和一定体积18.4mol•L-1的浓硫酸放在圆底烧瓶中共热,直到反应完毕,发现烧瓶中没有铜片剩余.往反应后的溶液中加入足量的BaCl2溶液,获得沉淀3.495g;产生的气体恰好使200mL 0.01mol•L-1的酸性高锰酸钾溶液褪色,则实验中取用的硫酸的物质的量为0.02mol.
分析 (1)先关闭止水夹E和分液漏斗旋塞,然后向分液漏斗中加水,再打开分液漏斗旋塞,根据分液漏斗中下端水的变化判断各装置的气密性;
(2)浓硫酸和铜在加热条件下发生反应生成硫酸铜、水和二氧化硫;反应中浓硫酸起到酸性和氧化性的作用;
(3)二氧化硫污染空气,应用氢氧化钠溶液吸收;
(4)装置B的作用是贮存多余的气体,所盛装溶液应避免二氧化硫的溶解和发生化学反应;
(5)生成沉淀为硫酸钡,根据S原子守恒可知反应后剩余的硫酸根离子的物质的量,再根据消耗的高锰酸钾的物质的量计算出二氧化硫的物质的量,然后根据质量守恒可知硫酸的物质的量.
解答 解:(1)检验各装置气密性是否良好的方法为:关闭止水夹E和分液漏斗的旋塞,向分液漏斗中加水,然后打开分液漏斗旋塞,若漏掉中的水滴入少量后停止,证明气密性良好,若水一直滴下,则气密性不好,
故答案为:关闭止水夹E和分液漏斗的旋塞,向分液漏斗中加水,然后打开分液漏斗旋塞,若漏掉中的水滴入少量后停止,证明气密性良好,若水一直滴下,则气密性不好;
(2)浓硫酸和铜在加热条件下发生反应生成硫酸铜、水和二氧化硫,反应的方程式为:Cu+2 H2SO4(浓)△_△––––––CuSO4+SO2↑+2 H2O,
故答案为:Cu+2 H2SO4(浓)△_△––––––CuSO4+SO2↑+2 H2O;
(3)二氧化硫污染空气,应该用氢氧化钠溶液吸收,可在棉花上浸有NaOH溶液,故答案为:吸收SO2气体,防止污染;
(4)二氧化硫易溶于水,可观察到集气瓶内液面下降,长颈漏斗内液面上升,装置B的作用是贮存多余的气体,所盛装溶液应避免二氧化硫的溶解和发生化学反应,则应选择饱和NaHSO3溶液,
故答案为:集气瓶内液面下降,长颈漏斗内液面上升;b;
(5)往反应后的溶液中加入足量的BaCl2溶液,获得沉淀3.495g,该沉淀为硫酸钡,其物质的量为:3.495g233g/mol3.495g233g/mol=0.015mol,说明反应后溶液中剩余0.015mol硫酸根离子;200mL 0.01mol•L-1的酸性高锰酸钾溶液中含有高锰酸钾的物质的量为:0.010mol/L×0.2L=0.002mol,根据关系式可2KMnO4~5SO2知,反应生成的二氧化硫的物质的量为:0.002mol×5252=0.005mol,根据S原子守恒可知浓硫酸中含有硫酸的物质的量为:0.015mol+0.005mol=0.02mol,
故答案为:0.02mol.
点评 本题考查浓硫酸的性质,题目难度中等,试题整合和拓展了教材中的典型实验,侧重基本实验操作能力考查,注意握常见气体的制备、除杂、收集、尾气处理等基本操作方法,熟悉教材中的典型实验装置,试题培养了学生的分析、理解能力及化学实验能力.

 学业测评一课一测系列答案
学业测评一课一测系列答案| A. |  表示某吸热反应分别在有、无催化剂的情兄下反应过程中的能量变化 | |
| B. | 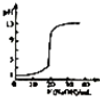 表示常温下,0.1 OOOmoi/LNaOH 溶液液滴定 20.00mL、0.0100/LHCl 溶液 所得到的滴定曲线 | |
| C. | 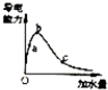 表示一定质量的冰醋酸加水稀释过程中,醋酸溶液电离程度:c>a>b | |
| D. | 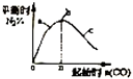 表示反应4CO+2NO2(g)?N2(g)+4CO2(g),在其他条件不变的情况下改变起始物CO的物质的量,平衡时N2的体识分数变化情况,由图可知NO2的转化率 c>b>a |