��Ŀ����
����Ŀ��ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr(OH)3��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2 �� O2��
��1���÷�Ӧ�еĻ�ԭ����_____��
��2���÷�Ӧ�У�������ԭ��Ӧ�Ĺ�����_____��______��
��3��д���÷�Ӧ�Ļ�ѧ����ʽ������˫���ŷ��������ת�Ƶķ������Ŀ______��
��4������Ӧת����0.3mol���ӣ�������������ڱ�״�������Ϊ______________L��
��5����֪���з��ӻ����������������¶�������KI�������������±仯��H2O2��H2O��MnO4����Mn2����IO3����I2��HNO2��NO������ֱ��õ����ʵ�������Щ��������������KI���õ�I2������_____��
A��H2O2 B��MnO4�� C��IO3�� D��HNO2
���𰸡�H2O2 H2CrO4 Cr(OH)3 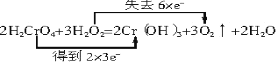 3.36 C
3.36 C
��������
H2O2��O2�Ĺ����У���Ԫ�صĻ��ϼ���-1��������0�ۣ����ݻ��ϼ��������������÷�Ӧ��CrԪ�صĻ��ϼ۽��ͣ�H2CrO4��Cr(OH)3�����ݻ��ϼ۵ı仯����Ӧ�ķ���ʽΪ2H2CrO4+3H2O2=2Cr(OH)3+3O2��+2H2O�����ݻ��ϼ۵ı仯�жϵ���ת�Ƶ���Ŀ���ݴ˷������
(1)H2O2��O2�Ĺ����У���Ԫ�صĻ��ϼ���-1��������0�ۣ�����H2O2�ǻ�ԭ�����ʴ�Ϊ��H2O2��
(2)��Ӧ��CrԪ�ػ��ϼ���Ҫ���ͣ�H2CrO4Ӧ����ԭ����Cr(OH)3���ʴ�Ϊ��H2CrO4��Cr(OH)3��
(3)��Ӧ�ķ���ʽΪ2H2CrO4+3H2O2=2Cr(OH)3+3O2��+2H2O����Ӧ��CrԪ�ػ��ϼ���+6�۽���Ϊ+3�ۣ�OԪ�ػ��ϼ���-1�����ߵ�0�ۣ���ת�Ƶ��ӵ���Ŀ�ͷ���ɱ�ʾΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(4)��2H2CrO4+3H2O2�T2Cr(OH)3��+3O2��+2H2O��֪������3mol����ת��6mol���ӣ���ת����0.3mol���ӣ����������������ʵ���Ϊ![]() mol��3=0.15mol���ڱ�״�������Ϊ0.15mol��22.4L/mol=3.36L���ʴ�Ϊ��3.36��
mol��3=0.15mol���ڱ�״�������Ϊ0.15mol��22.4L/mol=3.36L���ʴ�Ϊ��3.36��
(5)����I-ʱ��H2O2��H2O�õ�2�����ӣ�IO3-��I2�õ�5�����ӣ�MnO4-��Mn2+�õ�5�����ӣ�HNO2��NO�õ�1�����ӣ�I-��I2��ʧȥ1�����ӣ���IO3-����Ҳ����ԭΪI2����õ�I2������IO3-���ʴ�Ϊ��C��

����Ŀ�������ѣ�CH3OCH3���������������ʣ�����Ϊ21���͵������������Դ������δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�á���ҵ����CO��H2Ϊԭ������CH3OCH3���¹�����Ҫ����������Ӧ��
��CO��g��+2H2��g��CH3OH��g����H1=-91kJmol-1
��2CH3OH��g��CH3OCH3��g��+H2O��g����H2=-24kJmol-1
��CO��g��+H2O��g��CO2��g��+H2��g����H3=-41kJmol-1
�ش��������⣺
��1��д���¹��յ��ܷ�Ӧ���Ȼ�ѧ����ʽ��______
��2��ij�¶�����2L�����ܱ������м���CH3OH������Ӧ�ڣ�����й��������£�
��Ӧʱ��/min | 0 | 1 | 2 | 3 | 4 |
n��CH3OH��/mol | 1.02 | 0.42 | 0.22 | 0.02 | 0.02 |
�ٷ�Ӧ��2min����CH3OCH3��ʾ�Ļ�ѧ��Ӧ����Ϊ______
�ڸ��¶��µķ�Ӧ��ƽ�ⳣ��Ϊ______
��3��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ��ǰ��Ҫʹ�ڵ�λʱ�������CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��______
A ����ѹǿ B �������C ����CO2��Ũ�� D ������ϵ�¶�
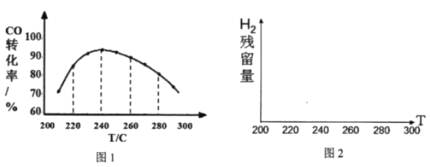
��4��Ϊ��Ѱ�Һ��ʵķ�Ӧ�¶ȣ��о��߽�����һϵ�����飬ÿ�����鱣��ԭ������ɡ�ѹǿ����Ӧʱ������ز��䣬������COת�������¶ȱ仯�Ĺ�����ͼ1���Խ���ԭ��______
��5�����о����ⶨ����ͬ�����������IJ�����������ͼ2�л���H2�IJ��������¶ȱ仯������______