��Ŀ����
����Ŀ�������������ڹ�ũҵ�����ж�����ҪӦ�á�
��1�������£�N2H4������������ĵ��⻯�
��֪��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ_________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=____����K1��K2��ʾ����
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ_________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=____����K1��K2��ʾ����
��2������2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����____������ĸ���ţ���
A��c(CO)=c(CO2) B�������л��������ܶȲ���
C��v(N2)��=2v(NO)�� D�������л�������ƽ��Ħ����������
��ͼ1Ϊ�����ڵ�ѹǿ��P������ʼѹǿ��P0���ı�ֵ��P/P0����ʱ�䣨t���ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)= ____��ƽ��ʱNO��ת����Ϊ____��

��3��ʹ�ü�ӵ绯ѧ���ɴ���ȼú�����е�NO��װ����ͼ2��ʾ����֪���ص�����������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ_________�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ��__________________��
���𰸡� 4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol ![]() D 0.006 mol��L��1��min��1 80% 2HSO3����2e����2H��=S2O42����2H2O 2NO��2S2O42����2H2O=N2��4HSO3��
D 0.006 mol��L��1��min��1 80% 2HSO3����2e����2H��=S2O42����2H2O 2NO��2S2O42����2H2O=N2��4HSO3��
��������(1)��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol��
���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol�� ![]() ��
��
(2)����2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��A��c(CO)=c(CO2)������ʾŨ�ȱ仯�������ж��Ƿ�Ϊƽ��״̬����A����B����Ӧ��������������䣬������䣬�����л��������ܶ�ʼ�ղ��䣬�����ж��Ƿ�Ϊƽ��״̬����B����C��v(N2)��=2v(NO)����ʾ��Ӧ����2v(N2)��=v(NO)�����ű�ʾ���淴Ӧ������ȣ���C����D���÷�Ӧ������������ʵ��������仯�ķ�Ӧ�������л�������ƽ��Ħ����������ʱ��ʾ��������ʵ������䣬 ˵����ƽ��״̬����D��ȷ����ѡD��
�����������ڵ�ѹǿ(P)����ʼѹǿ(P0)�ı�ֵ(P/P0)��ʱ��(t)�ı仯���ߣ�0��5min�ڣ� ![]() =0.925�����ݰ���٤�����ɼ������ۣ�
=0.925�����ݰ���٤�����ɼ������ۣ� ![]() =0.925��ƽ��ʱ
=0.925��ƽ��ʱ![]() =0.90��
=0.90��
2NO(g)�� 2CO(g) ![]() N2(g)��2CO2(g)
N2(g)��2CO2(g)
��ʼ(mol) 0.1 0.3 0 0
��Ӧ 2x 2x x 2x
5min��ƽ��
5minʱ�� ![]() =0.925�����x=0.03mol��v(N2)=
=0.925�����x=0.03mol��v(N2)= 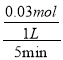 = 0.006mol��L��1��min��1��ƽ��ʱ��
= 0.006mol��L��1��min��1��ƽ��ʱ�� ![]() =0.90�����x=0.04mol��NO��ת����=
=0.90�����x=0.04mol��NO��ת����=![]() ��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
(3)����������ԭ��Ӧ����������������ӣ��õ��ӣ����������������ӣ��缫��ӦʽΪ��2HSO3-+2e-+2H+�TS2O42-+2H2O����������������һ����������������ԭ��Ӧ�����ɵ��������ӷ�Ӧ����ʽΪ��2NO+2S2O42-+2H2O�TN2+4HSO3-���ʴ�Ϊ��2HSO3-+2H++2e-=S2O42-+2H2O��2NO+2S2O42-+2H2O=N2+4HSO3-��


