��Ŀ����
����Ŀ���Ѽ��仯�����ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á�
(1)��̬��ԭ�ӵļ۵����Ų�ʽΪ_____________������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ����____________�֡�
(2)�ѱȸ��ᡢ����Ӳ����һ�����˵Ľṹ���ϣ��ѵ�Ӳ�ȱ������ԭ����_________��
(3)��Ũ��TiCl3��������Һ�м������ѣ���ͨ��HCl�����ͣ��ɵõ���λ��Ϊ6�����ΪTiCl3��6H2O����ɫ���壬�þ�����������������ʵ���֮��Ϊ1��5�����������ӵĻ�ѧʽΪ___________��
(4)����Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ľۺϣ���ṹ����ͼ��ʾ��
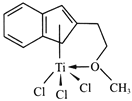
�����M��Ԫ���У��縺��������_________(������)��
��M��̼ԭ�ӵ��ӻ���ʽΪ____________��
��M���________(�����)��
a���м� b���Ҽ� c�����Ӽ� d����λ��
(5)���ʯ(TiO2)�Ǻ��ѵ���Ҫ����֮һ���侧���ṹ(��������ͬλ�õ�ԭ����ͬ)��ͼ��ʾ��

��A��B��C��D 4������������ԭ����________(�����)��
����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����D��ԭ������ΪD(0.19a��____��___)���������ļ���d=______(�ô���ʽ��ʾ)��
���𰸡� 3d24s2 3 Tiԭ�ӵļ۵�������Al�࣬��������ǿ [TiCl(H2O)5]2+ �� sp2 sp3 c BD 0.81a 0.5c ![]()
�����������������(1)��ԭ�Ӻ�����22�����ӣ����ݺ�������Ų�����д��̬��ԭ�ӵļ۵����Ų�ʽ����̬ԭ�ӵ�δ�ɶԵ�����Ϊ2������������δ�ɶԵ�����Ϊ2��Ԫ����Ge��Se��Ni����3����(2) Tiԭ�ӵļ۵�������4����ԭ�ӵļ۵�������3��(3).��λ��Ϊ6��������������ʵ���֮��Ϊ1��5��������������1����ԭ�ӡ�5��ˮ���ӣ�(4) �����M��Ԫ����Ti��C��H��O��Cl���ǽ�����Խǿ�縺��Խ��M����˫��̼�͵���̼ԭ���������۵���Ϊ�Ҽ���˫����1����������1��������������M�Ľṹͼ��������λ����(5) �ٸ��ݾ�̯ԭ�����й���ԭ��![]() ����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2�����������ݾ����ṹ����Dԭ�����ꣻ����ͼʾ��
����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2�����������ݾ����ṹ����Dԭ�����ꣻ����ͼʾ��![]()
������(1)��ԭ�Ӻ�����22�����ӣ���̬��ԭ�ӵļ۵����Ų�ʽΪ3d24s2����̬��ԭ�ӵ�δ�ɶԵ�����Ϊ2������������δ�ɶԵ�����Ϊ2��Ԫ�ػ���3�֣��ֱ���Ge��Se��Ni�� (2) Tiԭ�ӵļ۵�������4����ԭ�ӵļ۵�������3��Tiԭ�ӵļ۵�������Al�࣬��������ǿ�������ѵ�Ӳ�ȱ�������(3).��λ��Ϊ6��������������ʵ���֮��Ϊ1��5��������������1����ԭ�ӡ�5��ˮ���ӣ����Ը�������ӵĻ�ѧʽΪ[TiCl(H2O)5]2+��(4) �����M��Ԫ����Ti��C��H��O��Cl������O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խ�����Ե縺��������������M����˫��̼�͵���̼ԭ������������M��̼ԭ�ӵ��ӻ���ʽΪsp2�� sp3���۵���Ϊ�Ҽ���˫����1����������1��������������M�Ľṹͼ��������λ����û�����Ӽ�����ѡc��(5) �ٸ��ݾ�̯ԭ�����й���ԭ��![]() ����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2����֪��ԭ����BD�������ݾ����ṹ����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����Dԭ��������(0.19a��0.81a��0.5c)������ͼʾ��
����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2����֪��ԭ����BD�������ݾ����ṹ����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����Dԭ��������(0.19a��0.81a��0.5c)������ͼʾ��![]() ����d=
����d=![]() ��
��

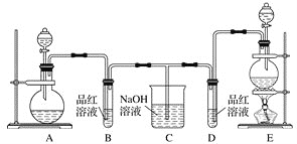
����Ŀ��������ʯ����Ҫ�ɷ�ΪFeS2������FeS(�������������в���������Ԫ�أ��Ҹ����²�������ѧ�仯)�������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС��Ըû�����ʯ��������ʵ��̽������m1g�û�����ʯ����Ʒ������ͼװ��(�гֺͼ���װ����)��ʯӢ���У���a�����ϵػ���ͨ��������������ջ�������Ʒ����Ӧ��ȫ���䷴Ӧ�Ļ�ѧ����ʽΪ4FeS2+11O2=2Fe2O3+8SO2��4FeS+7O2=2Fe2O3+4SO2
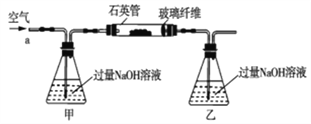
��ʵ��һ���ⶨ��Ԫ�صĺ���
��Ӧ��������ƿ�е���Һ�������´�����
(1)���������������_____________________��
(2)��Ӧ��������ƿ��Һ�м�������H2O2��Һ��Ŀ����___________(�û�ѧ����ʽ��ʾ)��H2O2���Կ�����һ�ֺ������ᣬд������Ҫ�ĵ��뷽��ʽΪ____________________��
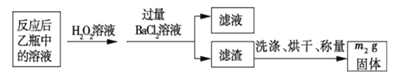
(3)�û���ɰʯ����Ԫ�ص���������Ϊ____________________(�г�����ʽ����)��
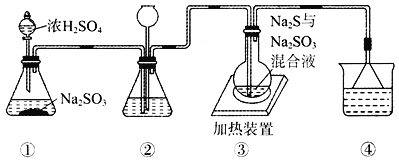
��ʵ������ⶨ��Ԫ�صĺ���
��������ϡ�����ܽ�ʯӢ���еĹ�������ڼӻ�ԭ��ʹ��Һ�е�Fe3+ǡ����ȫת��ΪFe2+���ˡ�ϴ�� �۽�����Һϡ����250mL
��ȡ25.00mLϡ��Һ����0.100mol��L-1������KMnO4��Һ�ζ�
(4)������У�������������ԭ����������õ���Ԫ�صĺ���__________(�ƫ�� ƫС�� ����Ӱ�족)��
(5)��д���������ϴ�ӵķ���____________________��
(6)ijͬѧһ���������Ĵεζ�ʵ�飬ʵ������¼���£�
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
����KMnO4��Һ���/ml | 25.00 | 25.03 | 20.00 | 24.97 |
�����������ݣ������ϡ��Һ��Fe2+�����ʵ���Ũ��c(Fe2+)=__________��