��Ŀ����
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�
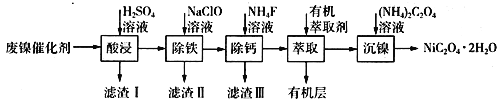
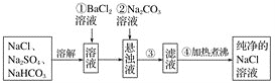
(1)��д��һ������ߡ���������ʵĴ�ʩ��________________________������I�ijɷ���____________(�ѧʽ)��
(2)����ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��
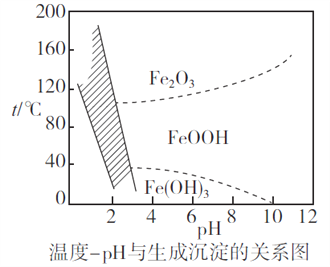
���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ_________________________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12](ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
(3)��֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_________mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
(4)�����л���ȡ����������________________________��
(5)ij��ѧ�����Լ��Ļ�ѧʽΪMxNi(SO4)y(MΪ+1�������ӣ�NiΪ+2�ۣ�x��y��Ϊ������)��Ϊ�ⶨ�ö����Լ�����ɣ���������ʵ�飺
I������28.7g�����Լ�������100 mL��ҺA��
��ȷ��ȡ10.00 mL��ҺA����0.40 mol��L-1��EDTA(Na2H2Y)����Һ�ζ����е�Ni2+(���ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+)������EDTA����Һ25.00mL��
����ȡ10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g��
������100 mL�����Լ�ʱ����Ҫ��������ҩ�ס�������ƽ�����������ձ�����Ͳ����ͷ�ι��⣬����Ҫ________________________��
�ڸö����Լ��Ļ�ѧʽΪ________________________________��
���𰸡� �ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ����� SiO2��CaSO4 FeOOH 2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+ 6.6��10-2 ��ȥ��Һ�е�Zn2+ 100mL����ƿ (NH4)2Ni(SO4)2
�����������������(1)����Ӱ�췴Ӧ���ʵ����ط�����ߡ���������ʵĴ�ʩ������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ��(2)��������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ�����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12������(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() ��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������
��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������![]() �ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
�ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
������(1)����Ӱ�췴Ӧ���ʵ����أ������¶ȡ��ѷ����������顢�ʵ��������Ũ�Ȼ���������������ߡ����������������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ����������I�ijɷ���SiO2��CaSO4��(2) �ٸ�������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ���FeOOH����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12��������Ӧ�����ӷ���ʽ��2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() �������c(NH4F)=c mol��L-1����
�������c(NH4F)=c mol��L-1����![]() =
=![]() ��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L
��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L![]() 0.4 mol��L-1=0.01mol ������
0.4 mol��L-1=0.01mol ������ ![]() ��MxNi(SO4)y����Է�������=
��MxNi(SO4)y����Է�������=![]() �� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96
�� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96![]() =287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��
=287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�