��Ŀ����
����Ŀ��������Ϊ����һ�������������仯�������������������й㷺��;��
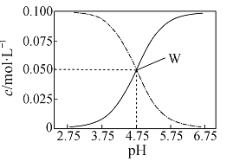
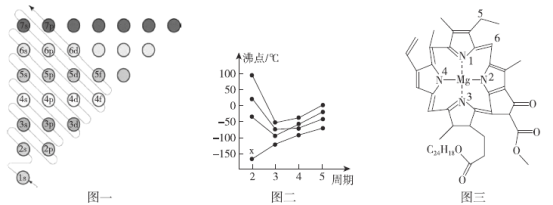
(1)��ԭ�Ӻ�����ӷ���ԾǨʱ�����ջ��ͷŲ�ͬ�Ĺ⣬������___________��ȡ��Ԫ�ص�ԭ�ӹ��ס�
(2)FeCl3���۵�Ϊ306�棬�е�Ϊ315�档�ɴ˿�֪FeCl3����______���塣FeSO4������ˮ���Ͳ�������SO42-�����幹����____________��
(3) ���軯��K3[Fe(CN)6]�Ǽ���Fe2+����Ҫ�Լ���
�ٻ�̬Nԭ�ӵĹ����ʾʽΪ___________��
��д��һ�������軯�������廥Ϊ�ȵ�����ļ��Է��ӵĻ�ѧʽ_______��
�����軯���У����漰��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ____________��
�����軯���У�������___________(����ĸ���)��
A.���Ӽ� B. ���� C. ���� D.��� E.������
(4)�л�������λ�������ï��[(C5H5)2Fe]�������еĿ�����������еĴ� �������÷�����mn��ʾ������m���������γɴ�������ԭ������ n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ��66)����![]() �еĴ�����Ӧ��ʾΪ________������̼ԭ�ӵ��ӻ���ʽΪ________��
�еĴ�����Ӧ��ʾΪ________������̼ԭ�ӵ��ӻ���ʽΪ________��
(5)�ʻ���[Fe(CO)5]���������������Ϳ������ȡ�1mol [Fe(CO)5]�����к�_____mol������
(6)ij�ִ��Ե������Ľṹ��ͼ��ʾ��N���������Fe���ɵ����� �����϶�С�������ԭ����Χ�������ԭ�Ӹ���Ϊ_____���������ױ߳�Ϊacm����Ϊc cm�������ӵ�������ֵΪNA����ô��Ե������ľ����ܶ�Ϊ_________g/cm3(�г�����ʽ)��

���𰸡������� ���� ���������� ![]() CO N��C��Fe��K DE ��
CO N��C��Fe��K DE ��![]() sp2 10 12
sp2 10 12 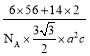
��������
(1) ��ͬԪ�ص�ԭ�ӷ���ԾǨʱ�����ջ��ͷŲ�ͬ�Ĺ⣬�����ù�������ȡ����Ԫ�صĵ��ӵ����չ�������ף����Կ����ù�������ȡ��Ԫ�ص�ԭ�ӹ��ף�
(2)FeCl3���۵�Ϊ306�棬�е�Ϊ315�棬����Խϵͣ��ɴ˿�֪FeCl3���ڷ��Ӿ��壻SO42-������ԭ��S��4���������Ӷԣ�������ԭ���ϵŶԵ��Ӷ���Ϊ��(6-2��4+2)/2=0������SO42-�����幹�������������Σ�
(3) ��̬Nԭ�ӵĹ����ʾʽΪ��![]() ��
��
�ڵȵ�������ָ�۵�������ԭ������ͬ�ķ��ӡ����ӻ�ԭ���ţ����軯��������(CN-)�۵�����Ϊ10�������以Ϊ�ȵ�����ļ��Է��ӵĻ�ѧʽ�У�CO��NO���ȣ�
��ÿ���ڵĵ�һ��Ԫ�صĵ�һ��������С�����һ��Ԫ�صĵ��������ͬ��Ԫ�ش��ϵ��µ�һ�����ܱ�С���������軯���У����漰��Ԫ��(Fe��C��N��K)�ĵ�һ�������ɴ�С��˳��Ϊ��N��C��Fe��K��
��K3[Fe(CN)6]�У�������д��ڵĻ�ѧ������������������ӵ����Ӽ���̼��֮��ļ��Թ��ۼ��������Ӻ�CN-֮�����λ�����������Ӽ䲻���������������ֻ���ڽ��������У����Ը��������û�н���������K3[Fe(CN)6]�в��������������������ѡDE��
(4)�������������֪��![]() �к��еĴ�����Ӧ��ʾΪ��
�к��еĴ�����Ӧ��ʾΪ��![]() ������̼ԭ�ӵļ۲���Ӷ���Ϊ3���¶Ե��ӣ��������ӻ���ʽΪsp2�ӻ���
������̼ԭ�ӵļ۲���Ӷ���Ϊ3���¶Ե��ӣ��������ӻ���ʽΪsp2�ӻ���
(5) 1��CO��������1��������1��CO������Fe�γ�1����λ������λ��Ҳ������������1mo1Fe(CO)5��10 mol ������
(6) �����������У� �۲��֪������̼ԭ����ṹ��Ԫ��6������Feԭ�����ڣ������ڲ�3��Feԭ��Ҳ���ڣ����ò�ΪABAB����ʽ���У�������ԭ���������ԭ�ӵĸ���Ϊ12���������ױ߳�Ϊa cm����Ϊc cm�������ӵ�������ֵΪNA����ô��Ե������ľ����ܶ�Ϊ��
�۲��֪������̼ԭ����ṹ��Ԫ��6������Feԭ�����ڣ������ڲ�3��Feԭ��Ҳ���ڣ����ò�ΪABAB����ʽ���У�������ԭ���������ԭ�ӵĸ���Ϊ12���������ױ߳�Ϊa cm����Ϊc cm�������ӵ�������ֵΪNA����ô��Ե������ľ����ܶ�Ϊ��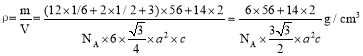 ��
��

����Ŀ����������Żش𣺢�HCl ��NaOH ��Cl2 ��H2O ��NH4Cl ��P4 ��NH3�� H2O ��Na2O2 ��HClO ��CaO HF MgCl2��
(1)ֻ�������Ӽ�����____________________
(2)���ڹ��ۻ��������____________________
(3)�ȴ������ӽ��ִ��ڹ��ۼ�����____________________
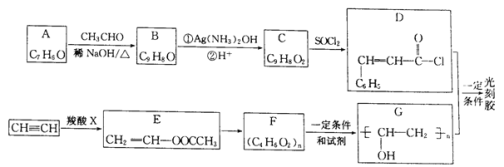
����ͬѧ���Ѿ�ѧϰ��ͬλ�ء�ͬϵ�ͬ�������塢ͬ���칹�壬����������Щ�����������г��˼������ʣ��뽫���ʵĺ��������д���±��С�
��![]() ��
��![]() �� ��CH3C(CH3)2CH3��
�� ��CH3C(CH3)2CH3��![]() ��
��
��CH4��CH3CH2CH3�� �ܽ��ʯ��ʯī����뭡����밣���16O��17O��18O�����Ҵ�(CH3CH2OH)�ͼ���(CH3OCH3)��������(O2)�����(O3)��
��� | ͬλ�� | ͬϵ�� | ͬ�������� | ͬ���칹�� |
��� | ________ | ___________ | ________ | _______ |