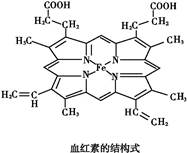
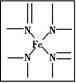
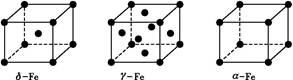
��Ŀ����
�ڻ�ҩ���й��Ŵ��Ĵ���֮һ�����ı�ը��ӦΪ��
2KNO3+3C+S A+N2��+3CO2��������ƽ����
A+N2��+3CO2��������ƽ����
��1���������г��˵����������̼�⣬����һ������A����A�ĵ���ʽΪ ��A����________�����
��2���ڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ���� �֡�����һ��Ԫ�صĵ��ʿ�������ҩ���������������Ư��֯���������������Ԫ�������ڱ��е�λ���� ��
��3���ڻ�ҩ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� ����Ԫ�ط��ű�ʾ����
��4��������ʵ��˵���ڻ�ҩ��̼������Ԫ�طǽ��������ǿ������ ��
A��ͬ����ͬŨ����Һ��pH��Na2CO3��Na2SO4
B�����ԣ�H2SO3��H2CO3
C��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2��
2KNO3+3C+S
 A+N2��+3CO2��������ƽ����
A+N2��+3CO2��������ƽ������1���������г��˵����������̼�⣬����һ������A����A�ĵ���ʽΪ ��A����________�����
��2���ڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ���� �֡�����һ��Ԫ�صĵ��ʿ�������ҩ���������������Ư��֯���������������Ԫ�������ڱ��е�λ���� ��
��3���ڻ�ҩ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� ����Ԫ�ط��ű�ʾ����
��4��������ʵ��˵���ڻ�ҩ��̼������Ԫ�طǽ��������ǿ������ ��
A��ͬ����ͬŨ����Һ��pH��Na2CO3��Na2SO4
B�����ԣ�H2SO3��H2CO3
C��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2��
��1��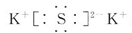 ����
����
��2��4 �������ڵڢ�A��
��3��K>S>C>N>O ��4��A��C
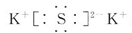 ����
������2��4 �������ڵڢ�A��
��3��K>S>C>N>O ��4��A��C
��1������ԭ���غ��֪AΪK2S�������ʽΪ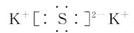 ���������ӻ����
���������ӻ����
��2���ڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ����C��N��O ��S���֡�������Ԫ��λ�����ڱ��е������ڵڢ�A�壬�䵥�ʿ�������ҩ��SO2������Ư��֯�������������
��3���ڻ�ҩ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����K>S>C>N>O��
��4��ͬ����ͬŨ����Һ��pH��Na2CO3��Na2SO4��˵������ H2SO4��H2CO3��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2�ۣ�˵����Ԫ��ԭ�ӵĵõ�������ǿ����������ʵ������˵����ķǽ����Ա�̼ǿ��B���У�H2SO3������Ԫ������������Ӧ��ˮ���
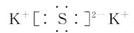 ���������ӻ����
���������ӻ������2���ڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ����C��N��O ��S���֡�������Ԫ��λ�����ڱ��е������ڵڢ�A�壬�䵥�ʿ�������ҩ��SO2������Ư��֯�������������
��3���ڻ�ҩ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����K>S>C>N>O��
��4��ͬ����ͬŨ����Һ��pH��Na2CO3��Na2SO4��˵������ H2SO4��H2CO3��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2�ۣ�˵����Ԫ��ԭ�ӵĵõ�������ǿ����������ʵ������˵����ķǽ����Ա�̼ǿ��B���У�H2SO3������Ԫ������������Ӧ��ˮ���

��ϰ��ϵ�д�
�����Ŀ