��Ŀ����
��֪A��B��C��D��Ϊ���ڱ���ǰ36��Ԫ�أ���ԭ��������������A��B��CΪͬһ���ڵ�����Ԫ�أ�Aԭ���������3��δ�ɶԵ��ӣ�Bԭ��p�ܼ�����������s�ܼ�����������ȡ�D�����ڱ�1��18���еĵ�10��Ԫ�ء���ش�
(1)C�ĵ����Ų�ͼΪ___________��
(2)A��B��Ԫ�صĵ�һ�����ܽϴ����____����дԪ�ط��ţ���
(3)A���⻯���VSEPRģ������ṹΪ__________��
(4)D����Ԫ�����ڱ��е�____����____��Ԫ�أ�����+2�����ӵĵ����Ų�ʽΪ__________��
(5)Ԫ��D��������DO����ṹ��NaCl����ṹ��ͬ����֪D2-�����O2-�ĺ˼����Ϊacm��DO��Ħ������ΪM g/mol�����á�NA����ʾ�����ӵ������� ��DO������ܶ�Ϊ_________��
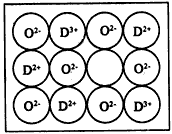
(6)��Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�ij��DO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��
D2+��ȱ����������D2+������D+��ȡ�������������Գʵ����ԣ�����������D��O�ı�ֵȴ�����˱仯��������D��Ʒ���ΪD0.97O����þ�����D3+��D2+�����Ӹ�����Ϊ____��
(1)C�ĵ����Ų�ͼΪ___________��
(2)A��B��Ԫ�صĵ�һ�����ܽϴ����____����дԪ�ط��ţ���
(3)A���⻯���VSEPRģ������ṹΪ__________��
(4)D����Ԫ�����ڱ��е�____����____��Ԫ�أ�����+2�����ӵĵ����Ų�ʽΪ__________��
(5)Ԫ��D��������DO����ṹ��NaCl����ṹ��ͬ����֪D2-�����O2-�ĺ˼����Ϊacm��DO��Ħ������ΪM g/mol�����á�NA����ʾ�����ӵ������� ��DO������ܶ�Ϊ_________��
(6)��Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�ij��DO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��
D2+��ȱ����������D2+������D+��ȡ�������������Գʵ����ԣ�����������D��O�ı�ֵȴ�����˱仯��������D��Ʒ���ΪD0.97O����þ�����D3+��D2+�����Ӹ�����Ϊ____��
(1)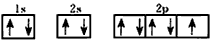
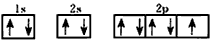
(2)N
(3)������
(4)�ģ�����1s22s22p63s23p63d8
(3)������
(4)�ģ�����1s22s22p63s23p63d8
(5)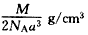
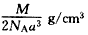
(6)Ni3+:Ni2+=6:91

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ




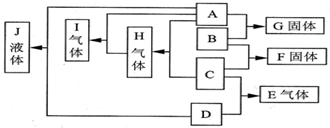

 ��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��


