��Ŀ����
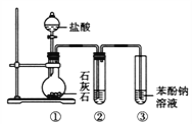
����Ŀ����.ʵ���ҳ��õļ������巢��װ����ͼA��B��C��ʾ��
�ش���������:
(1)����Aװ����Dװ��������ȡ���ռ�X���壬��X���������������е�_____������ѡ�
A.CO2 B.H2 C.Cl2
(2)Dװ�õ�С�ձ���Ӧʢ��NaOH��Һ��Ŀ����______��
(3)��Ҫ�ռ����﴿����X���壬����װ��Ӧ��θĽ�______��
(4)����Bװ����ȡ����,������_______________(���Լ�����)���и���.
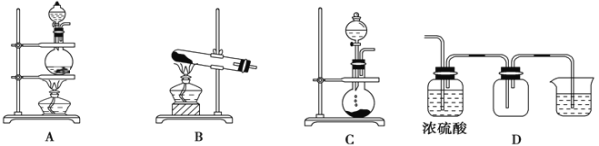
��.ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣(��֪��Na2SO3+H2SO4=Na2SO4+SO2��+H2O)
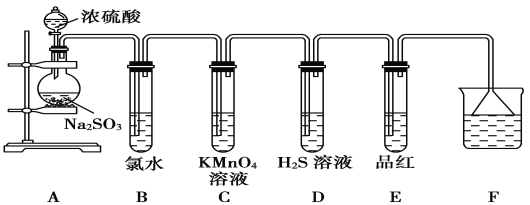
��ش��������⣺
(1)װ��A��ʢ��Ũ������������Ƶ��������Ʒֱ���________��________��
(2)��Ӧ��װ��B�з�����������______________ ��װ��D������SO2��________�ԣ�װ��E������SO2��________�ԡ�
(3)Fװ��©����������_____________________________��
(4)װ��C�з����ķ�Ӧ�����ӷ���ʽ_________________________��
���𰸡�C β�����д�������ֹ��Ⱦ���� ��װ��A��D֮������һ��ʢ�б���ʳ��ˮ��ϴ��ƿ ��ʯ��(��CaO��Ca(OH)2��) ��Һ©�� ��ƿ ����ɫ��dz ���� Ư�� ��ֹ���� 2MnO4-+5SO2+2H2O=5SO42-+2Mn2��+4H��
��������
I.����Aװ����Dװ��������ȡ���ռ�X���壬Ϊ������Һ�巴Ӧ��Ҫ����װ�ã������������Ũ�����������ſ������ռ�����Ҫβ��������
II.װ��A��ȡSO2��װ��B��C��֤SO2�Ļ�ԭ�ԣ�װ��D��֤�������ԣ�װ��E��֤��Ư���ԣ�װ��F�н���β�����������ݷ�Ӧǰ����Һ����ɫ��ͬ�жϷ�Ӧ����
I.(1)A��D������ȡ�����������������ռ����壬����װ��������Һ�����������ֹ��Ⱦ������
A.��ȡCO2ʱ����Ҫ����,Ҳ���ش���β����A���������⣻
B.H2��ȡ����Ҫ���ȣ������ռ�Ҫ�������ſ����ķ���������β������ʱһ����õ�ȼ�ķ�����B���������⣻
C.Cl2��ʵ���������ü���MnO2��Ũ�������﷽����ȡ���������ܶȱȿ������������ſ��������ռ��������ж���Ҫ��NaOH��Һ����β��������C�������⣻
�ʺ���ѡ����C��
(2)Cl2���ж����壬������ɴ�����Ⱦ������Ҫ��NaOH��Һ��β�����д��������ն������������ֹ��ɴ�����Ⱦ��
(3)ͨ��װ��A�ü���MnO2��Ũ�������﷽����ȡCl2������Ũ������лӷ��ԣ�������ȡ�������к���HCl��H2O��������Ҫ�ռ����﴿����X���壬Ӧ������NaCl������Һ��ȥ���е�HCl��Ȼ����Ũ��������Cl2�е�ˮ��������������װ����Ҫ�Ľ��ķ�������A��D֮������һ��ʢ�б���ʳ��ˮ��ϴ��ƿ������ȥ���к��е�HCl���ʣ�
(4)Bװ�������ڹ����������������ȡ���壬����Bװ����ȡ�����������Ǽ������壬Ҫ�ü��Ը�������ʯ�ҡ�����CaO�����Ca(OH)2������������и��
II. (1)װ��A�����ø��ֽⷴӦԭ����ȡSO2��Ũ����ʢ���ڷ�Һ©���У��������Ʒ�����ƿ�У�
(2)��װ��B�к������Ƶ���ˮ����Һ�Ի���ɫ��ͨ��SO2���壬������Ӧ��Cl2+SO2+2H2O=H2SO4+2HCl����Ӧ���������ᡢ���ᶼ����ɫ�ģ���˿���װ��B�з�������������Һ�Ļ���ɫ��dz������װ��D������Ӧ��SO2+2H2S=3S��+2H2O��SԪ�صĻ��ϼ���SO2�е�+4�۱�ΪS���ʵ�0�ۣ����ϼ۽��ͣ���õ��ӣ�����ԭ�����Ա�����SO2�������ԣ�װ��Eʢ��Ʒ����Һ���ῴ����Һ�ĺ�ɫ��Ϊ��ɫ��������SO2��Ư���ԡ�
(3)װ��F�а�װ��һ������©��������������SO2����Һ���������ʹSO2����Һ������գ���ֹ��������ķ�����
(4)װ��C��KMnO4��Һ����ǿ�������ԣ��ܽ�SO2����Ϊ���ᣬ�����ķ�Ӧ�����ӷ���ʽ��2MnO4-+5SO2+2H2O=5SO42-+2Mn2��+4H����