��Ŀ����
4��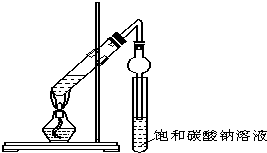 ijͬѧ����ͼ��ʾʵ��װ����ȡ�����������ش��������⣺
ijͬѧ����ͼ��ʾʵ��װ����ȡ�����������ش��������⣺��1����λͬѧ���������θ���ܴ����˳����ܣ���������ܵ�ĩ�˲����˱���̼������Һ�У��ڴ˴����θ���ܵ����ó���ʹ�����������������з�ֹ������
��2���мס��ҡ�����λͬѧ���ֱ��������Ҵ���Ӧ�õ�������δ�ñ���Na2CO3��Һ�нӣ��ᴿ����δ��ָʾ��������£����Ƕ����ȼ�NaOH�к����ᣬȻ������������������������ǵĽ��ȴ��ͬ��
�ټõ��˲�����ˮ����������
���ҵõ������Ե����Ļ���
�۱��õ�����ˮ�������ʣ�
�Է��������������������ԭ��
�ף�����NaOH��Һǡ���к��˹������ᣬ�õ���������
�ң�����NaOH��Һ����δ������ȫ��Ӧ
��������NaOH��Һ�������ڼ���������������ˮ�⣮
���� ��1�����θ���ܵĵ��ܿ�����Һ���¿��ܷ���������
��2������NaOH�����ᡢ��������Ӧ�Լ�NaOH���IJ�ͬ��Ӧ���еij̶Ȳ�ͬ��
��� �⣺��1�����θ���ܵ����ó���ʹ����������������⣬�����Է�ֹ�������ʴ�Ϊ����ֹ������
��2���ټõ��˲�����ˮ����������û���ᣬ˵������NaOH��Һǡ���к��˹������ᣬ�ʴ�Ϊ������NaOH��Һǡ���к��˹������ᣬ�õ�����������
���ҵõ������Ե����Ļ�������ʣ�࣬˵��������NaOH��Һ����δ������ȫ��Ӧ���ʴ�Ϊ������NaOH��Һ����δ������ȫ��Ӧ��
�۱��õ�����ˮ�������ʣ�˵��û����������Ϊ����NaOH��Һ������������ˮ�⣬�ʴ�Ϊ������NaOH��Һ�������ڼ���������������ˮ�⣮
���� ���⿼�������������Ʊ����ѶȲ��ڣ�2���������Һ�ijɷַ�����Ӧ���еij̶ȣ�������ѧ����������ͽ�������������

��ϰ��ϵ�д�
 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
�����Ŀ
19������ʵ��������ܴﵽʵ��Ŀ���ǣ�������
| A�� | �����Ը��������Һ������������ϩ | |
| B�� | ��������Һ�м���ϡ���ᣬ���ȣ���ȴ���������Cu��OH��2����ˮ����� | |
| C�� | �ñ���Na2CO3��Һ�������ᡢ�Ҵ��������� | |
| D�� | ����ȼ�յķ���������ë���� |
9�����й��ڳ����л����˵������ȷ���ǣ�������
| A�� | ��ϩ�ͱ�����ʹ���������Һ��ɫ | |
| B�� | �������֬����������������Һ��Ӧ | |
| C�� | ����͵����ʶ���������Ҫ��Ӫ������ | |
| D�� | ��ϩ�ͼ��������ˮ���� |
10������ʵ�������ԭ������ȷ���ǣ�������
| A�� | ֽ�������е�չ����֮�����ܹ�չ������Ҫԭ����ëϸ���� | |
| B�� | ����ʱ����ֽҪС�ڲ���©�� | |
| C�� | ��ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� | |
| D�� | �ⶨ��ҺpH�IJ�������pH��ֽ���ڱ������ϣ��ýྻ������պȡ��Һ������pH��ֽ���в������Ӧ�ı���ɫ���Ƚ� |
11�����л�ѧʽֻ����һ�����ʷ��ӵ��ǣ�������
| A�� | CH2Cl2 | B�� | C2H4Cl2 | C�� | C4H10 | D�� | C2H6O |
 2Z��g������X��Y��Z����ʼŨ�ȷֱ�Ϊ c1��c2��c3������Ϊ�㣩���ﵽƽ��ʱ��X��Y��Z��Ũ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.08mol/L���������ж���ȷ���ǣ� ��
2Z��g������X��Y��Z����ʼŨ�ȷֱ�Ϊ c1��c2��c3������Ϊ�㣩���ﵽƽ��ʱ��X��Y��Z��Ũ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.08mol/L���������ж���ȷ���ǣ� ��

 ��
��
 ��
��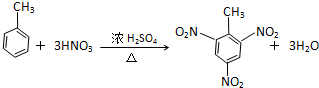 ��
��