��Ŀ����
����Ŀ���о����֣�NOx��SO2����������Ҫ�ɷ֡�
��һ��NOx��Ҫ��Դ������β����
��֪��N2��g����O2��g��![]() 2NO��g�� ��H����180.50 kJ��mol��1
2NO��g�� ��H����180.50 kJ��mol��1
2CO��g����O2��g��![]() 2 CO2��g�� ��H����566.00kJ��mol��1
2 CO2��g�� ��H����566.00kJ��mol��1
��1��Ϊ�˼��������Ⱦ���������������β�������ܿڲ��ô�����NO��COת��������Ⱦ����������ѭ����д���÷�Ӧ���Ȼ�ѧ����ʽ____________��
��2��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2L���ܱ������У������¶Ⱥ�������䣬��Ӧ���̣�0��15min����NO�����ʵ�����ʱ��仯��ͼ��ʾ��

��T��ʱ�û�ѧ��Ӧ��ƽ�ⳣ��K��_________��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8 mol��ƽ�⽫_______�ƶ���������������ҡ�������
��ͼ��a��b�ֱ��ʾ��һ���¶��£�ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n��NO���ı仯���ߣ����б�ʾ����������ϴ��������__________�����a����b����
��15minʱ�����ı���練Ӧ����������n��NO��������ͼ��ʾ�ı仯����ı������������____________��
������SO2��Ҫ��Դ��ú��ȼ�ա�ȼú��������������Ǽ��ٴ����к�������Ⱦ�Ĺؼ���
��3���ô�����Һ����SO2�ɽ���ת��ΪHSO3-���÷�Ӧ�����ӷ���ʽ��_____________��
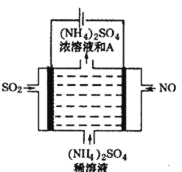
��4����ͼ���װ�ÿɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42-��
��д������A�Ļ�ѧʽ_____________�������ĵ缫��Ӧʽ��____________Cl��
�ڸõ�ⷴӦ�Ļ�ѧ����ʽΪ________________��
���𰸡���1��2NO��g��+2CO��g��=2CO2��g��+N2��g�� ��H =-746.50 kJ��mol��1
��2����5(mol/L)��1 ����
��b ������CO�����ʵ���Ũ�Ȼ�����ѹǿ
��3��H2O+2SO2+CO32-��2HSO3��+CO2��
��4����H2SO4 SO2+2H2O-2e����SO42-+4H+
��5SO2+2NO+8H2O![]() (NH4)2SO4+4H2SO4
(NH4)2SO4+4H2SO4
��������
�����������1����֪���� N2��g����O2��g��![]() 2NO��g�� ��H����180.50 kJ��mol��1
2NO��g�� ��H����180.50 kJ��mol��1
�� 2CO��g����O2��g��![]() 2 CO2��g�� ��H����566.00kJ��mol��1
2 CO2��g�� ��H����566.00kJ��mol��1
���ݸ�˹�������������õ�2NO��g��+2CO��g��=2CO2��g��+N2��g�� ��H =-746.50 kJ��mol��1��
��2������ʼʱ��NOΪ0.4mol��ƽ��ʱNOΪ0.2mol����
2NO��g��+2CO��g��=2CO2��g��+N2��g��
��ʼ���ʵ�����0.4mol 0.4mol 0 0
ת������������0.2mol 0.2mol 0.2mol 0.1mol
ƽ�����ʵ�����0.2mol 0.2mol 0.2mol 0.1mol
��ƽ��ʱ��Ũ�ȣ�c��NO��=0.1mol/L��c��CO��=0.1mol/L��c��CO2��=0.1mol/L��c��N2��=0.05mol/L��
����ƽ�ⳣ��K��![]() =5��mol/L��-1��
=5��mol/L��-1��
ƽ��ʱ�������¶Ȳ��������������г���CO��N2��0.8mol����c��CO��=0.5mol/L��c��N2��=0.45mol/L��
Qc��![]() ��1.8��5����ƽ�⽫�����ƶ���
��1.8��5����ƽ�⽫�����ƶ���
������������ϴ�Ӧ���ʿ죬�ﵽƽ������ʱ��̣���ͼ��֪��b���ߴ����������·�Ӧ���ʿ죬����b�Ĵ����ı������
����ͼ���֪��NO��Ũ�ȼ�С��ƽ�����������ƶ������Ըı������Ϊ����CO�����ʵ���Ũ�Ȼ�����ѹǿ��
��3��̼������Һ��SO2��Ӧ�������������ƺͶ�����̼���䷴Ӧ�����ӷ���ʽΪH2O+2SO2+CO32-��2HSO3��+CO2����
��4�����װ�D�ɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42-�����ⷽ��ʽΪ5SO2 + 2NO + 8H2O ![]() (NH4)2SO4 + 4H2SO4����
(NH4)2SO4 + 4H2SO4����
���ɵ�ⷽ��ʽ��֪������AΪ���ᣬ��Ļ�ѧʽH2SO4�� ���ʱ�������϶�������ʧ����������������ӣ��������ĵ缫��Ӧʽ��SO2+2H2O-2e����SO42-+4H+��
���������Ϸ�����֪�õ�ⷴӦ�Ļ�ѧ����ʽΪ5SO2 + 2NO + 8H2O![]() ( NH4)2SO4 + 4H2SO4��
( NH4)2SO4 + 4H2SO4��

����Ŀ��I��ijѧ����0.2000 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
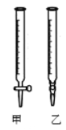
��������ˮϴ�Ӽ�ʽ�ζ�������ע��NaOH��Һ����0���̶�������
���̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
������Һ������0����0���̶������£������¶���
����ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�����
��ش�
��1�����ϲ����д������ǣ����ţ� ��
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��______��������ͼ��ѡ��ס����ҡ���
��3�����в���������ʵ����ƫ����ǣ�______�����ţ�
A������ƿװҺǰ��������������ˮ
B���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ
D������ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ
��4���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע�� ���жϵ���ζ��յ�������ǣ���ƿ����Һ ��
��5��������ʵ�����ݼ�¼��
�ζ����� | �������mL | NaOH��Һ���������mL�� | |
�ζ�ǰ | �ζ��� | ||
1 | 20.00 | 0.00 | 21.30 |
2 | 20.00 | 0.00 | 16.30 |
3 | 20.00 | 0.00 | 16.32 |
ͨ������ɵã�������Ũ��Ϊ��______ molL-1������������4λС����
II�����ü�����ζ����ɲⶨBa2���ĺ�����ʵ����������С�
��֪��2CrO42����2H����Cr2O72����H2O Ba2����CrO42����BaCrO4��
����1����ȡx mLһ��Ũ�ȵ�Na2CrO4��Һ����ƿ�У��������ָʾ������b mol��L��1�����Һ�ζ����յ㣬��õμ��������ΪV0 mL��
����2����ȡy mL BaCl2��Һ����ƿ�У�����x mL�벽��:1��ͬŨ�ȵ�Na2CrO4��Һ����Ba2����ȫ�������ټ������ָʾ������b mol��L��1�����Һ�ζ����յ㣬��õμ���������ΪV1 mL��
��BaCl2��ҺŨ��Ϊ______________________ mol��L��1��������2�еμ�����ʱ����������Һ��������Ba2��Ũ�ȵIJ���ֵ��___________���ƫ��ƫС������