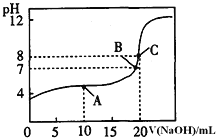
��Ŀ����
����Ŀ���������£�����������Һ��
��0.1mol/L NH4Cl ��0.1mol/L CH3COONH4 �� 0.1mol/L NH4HSO4
��0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ ��0.1mol/L NH3H2O
�����Ҫ����д���пհף�
��1����Һ�ٳ�_________�ԣ���ᡱ��������С�������ԭ����_____________�������ӷ���ʽ��ʾ����
��2��������������Һ�У�pH��С����_____��c��NH4+����С����____������ţ���
��3������Һ���У�_____________���ӵ�Ũ��Ϊ0.1mol/L��NH3H2O��________���ӵ����ʵ���Ũ��֮��Ϊ0.2 mol/L��
��4�������£������Һ�ڵ�pH=7����CH3COO����NH4+Ũ�ȵĴ�С��ϵ�ǣ�c��CH3COO����_____________c��NH4+�������������������������
���𰸡��� NH4++H2O![]() H++NH3H2O �� �� Cl- NH4+ =
H++NH3H2O �� �� Cl- NH4+ =
��������
����ˮ��һ�������ģ�����Ӱ��������ˮ�����ӵ����أ����������ʵĵ��룬�ж���Һ��������Լ��������Ũ�ȵĴ�С��HSO4-�������H+����NH4+��ˮ�⣻�����ʵ���Ũ�ȵ�NH3H2O��0.1mol/L NH4Cl���Һ��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ�NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ��
��1��NH4Cl��ǿ�������Σ�NH4+ˮ�������ԣ�NH4+ˮ�ⷴӦ�����ӷ���ʽNH4++H2O![]() H++NH3H2O��
H++NH3H2O��
��2������Һ��������Ũ��Խ����ҺpHԽС����0.1mol/L NH4Cl��NH4+ˮ�������ԣ�Cl-��ˮ�⣻��CH3COONH4�����������Σ�0.1mol/L CH3COONH4��NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ����Һ�����ԣ��� 0.1mol/L NH4HSO4��HSO4- = H++SO42-��HSO4-�������H+����NH4+��ˮ�⣻��0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ���Һ��NH4+ˮ���Ũ�ȴ���0.1mol/L����0.1mol/L NH3H2O��NH3H2O��������ʣ�NH3H2O![]() NH4++OH-�� NH3H2O����������NH4+��������������֪��ҺpH��С���Ǣ� 0.1mol/L NH4HSO4��c(NH4+)��С���Ǣ�0.1mol/L NH3H2O��
NH4++OH-�� NH3H2O����������NH4+��������������֪��ҺpH��С���Ǣ� 0.1mol/L NH4HSO4��c(NH4+)��С���Ǣ�0.1mol/L NH3H2O��
��3����0.1mol/L NH3H2O��0.1mol/L NH4Cl���Һ�������ӵ����ʵ������䡢Ũ�Ȳ��䣬�����ӵ�Ũ��Ϊ0.1mol/L��NH3H2O�ĵ������NH4+ˮ�⣬��Һ�ʼ��ԣ��������غ��֪��c(NH3H2O)+c(NH4+)=0.2 mol/L��
��4�������£������Һ�ڵ�pH=7��NH4+��CH3COO-��ˮ�⣬����ˮ����ٽ��̶���ͬ��c(CH3COO-)=c(NH4+)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�