题目内容
已知下列热化学方程式
(l)C(s)+
O2(g)═CO(g)△H1=-110.5kJ/mol
(2)2H2(g)+O2(g)═2H2O(g)△H2=-483.6kJ?mol-1
由此可知C(s)+H2O(g)═CO(g)+H2(g)△H3.则△H3等于( )
(l)C(s)+
| 1 |
| 2 |
(2)2H2(g)+O2(g)═2H2O(g)△H2=-483.6kJ?mol-1
由此可知C(s)+H2O(g)═CO(g)+H2(g)△H3.则△H3等于( )
| A.+131.3kJ?mol-1 | B.-131.3kJ?mol-1 |
| C.+373.1kJ?mol-1 | D.-373.1kJ?mol-1 |
由(l) C(s)+1/2O2(g)=CO(g)△H1=-110.5kJ/mol,
(2)2H2(g)+O2(g)=2H2O(g)△H2=-483.6kJ?mol-1,
依据盖斯定律可知,(1)-(2)÷2即得到:C(s)+H2O(g)═CO(g)+H2(g),所以△H3=-110.5kJ/mol+483.6kJ/mol÷2=+131.3kJ/mol,
故选A.
(2)2H2(g)+O2(g)=2H2O(g)△H2=-483.6kJ?mol-1,
依据盖斯定律可知,(1)-(2)÷2即得到:C(s)+H2O(g)═CO(g)+H2(g),所以△H3=-110.5kJ/mol+483.6kJ/mol÷2=+131.3kJ/mol,
故选A.

练习册系列答案
 优百分课时互动系列答案
优百分课时互动系列答案 开心蛙状元作业系列答案
开心蛙状元作业系列答案 课时掌控随堂练习系列答案
课时掌控随堂练习系列答案
相关题目
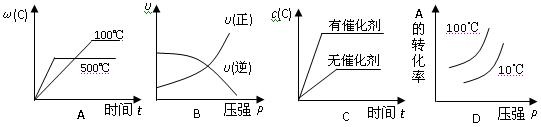
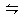 2NO2(g)
2NO2(g)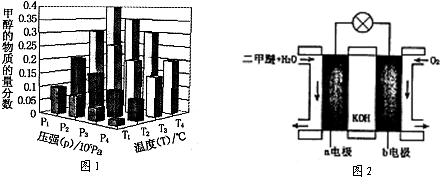
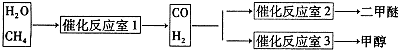
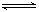 2C(g) △H<0,下列各图正确的是( )
2C(g) △H<0,下列各图正确的是( )