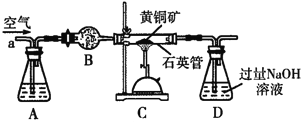
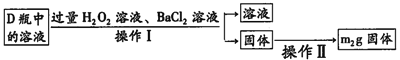
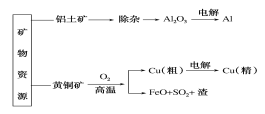
ЬтФПФкШн
ЁОЬтФПЁПЂё.ЃЈ1ЃЉецПеЬМШШЛЙд-бѕЛЏЗЈПЩЪЕЯжгЩТСПѓжЦБИН№ЪєТСЃЌЦфЯрЙиЕФШШЛЏбЇЗНГЬЪНШчЯТЃК
Al2O3(s)+AlCl3(g)+3C(s)ЃН3AlCl(g)+3CO(g) ЁїH=akJЁЄmol-1
3AlCl(g)ЃН2Al(l)+AlCl3(g) ЁїH=bkJЁЄmol-1
ЗДгІAl2O3(s)+3C(s)ЃН2Al(l)+3CO(g)ЕФЁїH=___kJЁЄmol-1(гУКЌaЁЂbЕФДњЪ§ЪНБэЪО)ЃЛ
ЃЈ2ЃЉвбжЊ2SO2(g)+O2(g)=2SO3(g) ЁїHЗДгІЙ§ГЬЕФФмСПБфЛЏШчЭМЫљЪОЃЌвбжЊ1molSO2(g)бѕЛЏЮЊ1molSO3ЗХГі99kJЕФШШСПЃЌЧыЛиД№ЯТСаЮЪЬтЃК
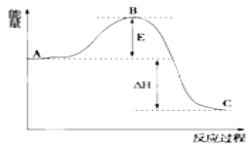
ЂйЭМжаAЗжБ№БэЪО____ЃЛ
ЂкEЕФДѓаЁЖдИУЗДгІЕФЗДгІШШ____(ЬюЁАгаЁБЛђЁАЮоЁБ)гАЯьЃЛ
ЂлИУЗДгІЭЈГЃгУV2O5зїДпЛЏМСЃЌМгV2O5ЛсЪЙЭМжаBЕу____(ЬюЁАЩ§ИпЁБЁАНЕЕЭЁБ)ЁЃ
Ђђ.ЬМЪЧаЮГЩЛЏКЯЮяжжРрзюЖрЕФдЊЫиЃЌЦфЕЅжЪМАЛЏКЯЮяЪЧШЫРрЩњВњЩњЛюжаЕФжївЊФмдДЮяжЪЁЃЧыЛиД№ЯТСаЮЪЬтЃК
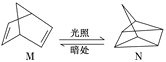
ЃЈ3ЃЉгаЛњЮяMОЙ§ЬЋбєЙтЙтееПЩзЊЛЏГЩNЃЌзЊЛЏЙ§ГЬШчЭМЃК
 ІЄHЃНЃЋ88.6 kJ/mol
ІЄHЃНЃЋ88.6 kJ/mol
дђMЁЂNЯрБШЃЌНЯЮШЖЈЕФЪЧ___ЁЃ
ЃЈ4ЃЉвбжЊCH3OH(l)ЕФШМЩеШШІЄHЃНЃ238.6kJ/molЃЌCH3OH(l)ЃЋ3/2O2(g)ЃНCO2(g)ЃЋ2H2O(g) ІЄHЃНЃakJ/molЃЌдђa__238.6(ЬюЁАЃОЁБЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
ЁОД№АИЁПa+b ЗДгІЮязмФмСП Юо НЕЕЭ M ЃМ
ЁОНтЮіЁП
ЃЈ1ЃЉИљОнИЧЫЙЖЈТЩЧѓНтЃЛ
ЃЈ2ЃЉьЪБфЮЊЩњГЩЮяЕФзмФмСП-ЗДгІЮяЕФзмФмСПЃЌДпЛЏМСЖдЗДгІЕФьЪБфЮогАЯьЃЌжЛгАЯьЗДгІЕФЛюЛЏФмЃЛ
ЃЈ3ЃЉЮяжЪОпгаЕФФмСПдНЕЭдНЮШЖЈЃЛ
ЃЈ4ЃЉРћгУH2O(g)=H2O(l)ЗХГіШШСПНјааЧѓНтЁЃ
ЃЈ1ЃЉИљОнИЧЫЙЖЈТЩЃЌЩЯЪіСНЪНЯрМгМДПЩЕУЕНAl2O3(s)+3C(s)ЃН2Al(l)+3CO(g)ЃЌдђЁїH=(a+b)kJЁЄmol-1ЃЛ
ЃЈ2ЃЉЂйЭМжаAЮЊЗДгІЮяЕФзмФмСПЃЛ
ЂкьЪБфЮЊЩњГЩЮяЕФзмФмСП-ЗДгІЮяЕФзмФмСПЃЌгыEЕФДѓаЁЮоЙиЃЛ
ЂлДпЛЏМСФмЙЛНЕЕЭЗДгІЮяЕФЛюЛЏФмМДEЕФДѓаЁЃЌдђДпЛЏМСЛсЪЙBЕуНЕЕЭЃЛ
ЃЈ3ЃЉгаЛњЮяMОЙ§ЬЋбєЙтЙтееПЩзЊЛЏГЩNЃЌьЪБфЮЊ+88kJ/molЃЌЮЊЮќШШЗДгІЃЌдђNОпгаЕФФмСПДѓгкMЃЌMНЯЮШЖЈЃЛ
ЃЈ4ЃЉCH3OH(l)ЕФШМЩеШШЗНГЬЪНЮЊCH3OH(l)ЃЋ3/2O2(g)ЃНCO2(g)ЃЋ2H2O(l) ІЄHЃНЃ238.6 kJ/molЃЌгЩгкЦјЬЌЫЎЕФФмСПИпгквКЬЌЫЎЕФФмСПЃЌМДШМЩеЪБВњЩњЦјЬЌЫЎЪБЪЭЗХЕФШШСПЩйЃЌдђaЃМ238.6ЁЃ

ЁОЬтФПЁПCO2ЪЧвЛжжЮТЪвЦјЬхЃЌОнПЦбЇМвдЄВтЃЌЕН21ЪРМЭжавЖЃЌШЋЧђЦјЮТНЋЩ§Ип1.5ЁЋ4.5 ЁцЃЌЕиЧђЦјЮТЕФЩ§ИпЛсв§Ц№КЃЦНУцЩ§ИпЃЌЖдШЫРрЕФЩњДцЛЗОГВњЩњОоДѓЕФгАЯьЁЃШчКЮКЯРэЕиРћгУCO2ЪЧАкдкПЦбЇМвУцЧАЕФвЛИіжиДѓПЮЬтЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЙЄвЕЩЯРћгУИпЮТЁЂИпбЙЬѕМўЃЌПЩгУCO2гыNH3КЯГЩФђЫи[CO(NH2)2]ЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___ЁЃ
ЃЈ2ЃЉвЛЖЈЬѕМўЯТЃЌВЛЭЌСПЕФCO2гыВЛЭЌСПЕФNaOHГфЗжЗДгІЗХГіЕФШШСПШчЯТБэЫљЪОЃК
CO2ЕФСП | NaOHШмвКЕФСП | ЗХГіЕФШШСП | |
Ђй | 22.0 g | 750 mL 1.0 molЁЄLЃ1 | x kJ |
Ђк | 1.0 mol | 2.0 L 1.0 molЁЄLЃ1 | y kJ |
аДГіИУЬѕМўЯТCO2гыNaOHШмвКЗДгІЩњГЩNaHCO3ЕФШШЛЏбЇЗНГЬЪНЃК ___________________ЁЃ
ЃЈ3ЃЉдквЛЖЈЮТЖШКЭДпЛЏМСзїгУЯТЃЌПЩНЋCO2зЊЛЏЮЊШМСЯCH4ЃЌЗДгІЗНГЬЪНЮЊCO2(g)ЃЋ4H2(g) ![]() CH4(g)ЃЋ2H2O(g)ЁЁІЄHЁЃЕБ300 ЁцЪБЃЌвЛЖЈСПЕФCO2КЭH2ЛьКЯЦјЬхдкШнЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжаЗЂЩњЩЯЪіЗДгІЃЌ5 minКѓДяЕНЦНКтЃЌДЫЪБИїЮяжЪЕФХЈЖШШчЯТБэЃК
CH4(g)ЃЋ2H2O(g)ЁЁІЄHЁЃЕБ300 ЁцЪБЃЌвЛЖЈСПЕФCO2КЭH2ЛьКЯЦјЬхдкШнЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжаЗЂЩњЩЯЪіЗДгІЃЌ5 minКѓДяЕНЦНКтЃЌДЫЪБИїЮяжЪЕФХЈЖШШчЯТБэЃК
ЮяжЪ | CO2(g) | H2(g) | CH4(g) | H2O(g) |
ХЈЖШ/molЁЄLЃ1 | 0.2 | 0.8 | a | 1.6 |
дђЦНКтЪБШнЦїжаМзЭщЕФЮяжЪЕФСПn(CH4)ЃН________ЁЃДгЗДгІПЊЪМЕНДяЕНЦНКтЪБЕФЛЏбЇЗДгІЫйТЪv(CH4)ЃН__________ЁЃ500 ЁцЪБИУЗДгІЕФЦНКтГЃЪ§KЃН16ЃЌдђИУЗДгІЕФІЄH__________(ЬюЁАЃОЁБЁАЃМЁБ)0ЁЃ
ЃЈ4ЃЉCO2ЛЙПЩгУгкЩњВњМзДМЃЌвЛЖЈЬѕМўЯТЃЌЗЂЩњЗДгІCO2(g)ЃЋ3H2(g) ![]() CH3OH(g)ЃЋH2O(g)ЁЁІЄHЁЃ
CH3OH(g)ЃЋH2O(g)ЁЁІЄHЁЃ
ЂйдкШнЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжаЃЌЭЈШы2 mol CO2КЭ3 mol H2ЗЂЩњЩЯЪіЗДгІЃЌЯТСаЫЕЗЈФмЙЛБэУїИУПЩФцЗДгІДяЕНЦНКтзДЬЌЕФЪЧ__________(ЬюзжФИ)ЁЃ
aЃЎЯћКФ3 mol H2(g)ЪБЃЌга1 mol CH3OH(g)ЩњГЩ
bЃЎзЊвЦ3 molЕчзгЪБЃЌЗДгІЕФCO2ЮЊ11.2 L(БъзМзДПі)
cЃЎЬхЯЕжаЦјЬхЕФУмЖШВЛБф
dЃЎЫЎеєЦјЕФЬхЛ§ЗжЪ§БЃГжВЛБф
eЃЎЕЅЮЛЪБМфФкЩњГЩH2(g)гыЩњГЩH2O(g)ЕФЮяжЪЕФСПжЎБШЮЊ3ЁУ1
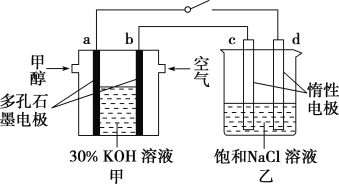
ЂкгУЖрПзЪЏФЋзїЕчМЋЃЌ30% KOHШмвКзїЕчНтжЪШмвКЃЌПЩЩшМЦШчЭММзЫљЪОЕФМзДМШМСЯЕчГиЃЌИУЕчГиЕФИКМЋЗДгІЪНЮЊ______________________________ЁЃШєНЋИУШМСЯЕчГигыЕчНтБЅКЭЪГбЮЫЎЕФзАжУНјааДЎСЊ(ШчЭМ)ЃЌЕБга0.12 mol ЕчзгЗЂЩњзЊвЦЪБЃЌЖЯПЊЕчдДЃЌНЋШмвКРфШДжСЪвЮТЃЌВтЕУЪГбЮШмвКЮЊ120 mLЃЌдђДЫЪБввзАжУжаШмвКЕФpHЃН________(МйЩшЪГбЮЫЎжагазуСПЕФNaClЃЌЧвCl2ЭъШЋвнГі)ЁЃ
ЁОЬтФПЁПФГЛЏбЇаЁзщЭЌбЇгУЯТСазАжУКЭЪдМСНјааЪЕбщЃЌЬНОПO2гыKIШмвКЗЂЩњЗДгІЕФЬѕМўЁЃ
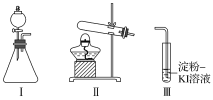
ЙЉбЁЪдМСЃК30%H2O2ШмвКЁЂ0.1mol/L H2SO4ШмвКЁЂMnO2ЙЬЬхЁЂKMnO4ЙЬЬх
ЃЈ1ЃЉаЁзщЭЌбЇЩшМЦМзЁЂввЁЂБћШ§зщЪЕбщЃЌМЧТМШчЯТ
Вйзї | ЯжЯѓ | |
Мз | ЯђIЕФзЖаЮЦПжаМгШы___ЃЌЯђIЕФ____жаМгШы30%H2O2ШмвКЃЌСЌНгIЁЂЂѓЃЌДђПЊЛюШћ | IжаВњЩњЮоЩЋЦјЬхВЂАщЫцДѓСПАзЮэЃЛЂѓжагаЦјХнУАГіЃЌШмвКбИЫйБфРЖ |
вв | ЯђЂђжаМгШыKMnO4ЙЬЬхЃЌСЌНгЂђЁЂЂѓЃЌЕуШМОЦОЋЕЦ | ЂѓжагаЦјХнУАГіЃЌШмвКВЛБфРЖ |
Бћ | ЯђЂђжаМгШыKMnO4ЙЬЬхЃЌЂѓжаМгШыЪЪСП0.1mol/LH2SO4ШмвКЃЌСЌНгЂђЁЂЂѓЃЌЕуШМОЦОЋЕЦ | ЂѓжагаЦјХнУАГіЃЌШмвКБфРЖ |
ЃЈ2ЃЉБћЪЕбщжаO2гыKIШмвКЗДгІЕФРызгЗНГЬЪНЪЧ____ЁЃ
ЃЈ3ЃЉЖдБШввЁЂБћЪЕбщПЩжЊЃЌO2гыKIШмвКЗЂЩњЗДгІЕФЪЪвЫЬѕМўЪЧ___ЁЃЮЊНјвЛВНЬНОПИУЬѕМўЖдЗДгІЫйТЪЕФгАЯьЃЌПЩВЩШЁЕФЪЕбщДыЪЉЪЧ___ЁЃ
ЃЈ4ЃЉгЩМзЁЂввЁЂБћШ§ЪЕбщЭЦВтЃЌМзЪЕбщПЩФмЪЧIжаЕФАзЮэЪЙШмвКБфРЖЁЃбЇЩњНЋIжаВњЩњЕФЦјЬхжБНгЭЈШыЯТСа_____(ЬюзжФИ)ШмвКЃЌжЄУїСЫАзЮэжаКЌгаH2O2ЁЃ
AЃЎЫсадKMnO4 BЃЎFeC12 CЃЎNa2S DЃЎЦЗКь
ЁОЬтФПЁП(1)вбжЊKsp[Cu(OH)2]ЃН2.2ЁС10Ѓ20ЃЌKsp[Fe(OH)3]ЃН2.6ЁС10Ѓ39ЁЃГЃЮТЯТЃЌФГЫсадCuCl2ШмвКжаКЌгаЩйСПЕФFeCl3ЃЌЮЊСЫЕУЕНДПОЛЕФCuCl2ЁЄ2H2OОЇЬхЃЌгІМгШы___________(ЬюбѕЛЏЮяЕФЛЏбЇЪН)ЃЌЕїНкШмвКЕФpHЃН4ЃЌЪЙШмвКжаЕФFe3ЃЋзЊЛЏЮЊFe(OH)3ГСЕэЃЌДЫЪБШмвКжаЕФc(Fe3ЃЋ)ЃН________ЁЃЙ§ТЫКѓЃЌНЋЫљЕУТЫвКЕЭЮТеєЗЂЁЂХЈЫѕНсОЇЃЌПЩЕУЕНCuCl2ЁЄ2H2OОЇЬхЁЃ
(2)ФГЬМЫиИжЙјТЏФкЫЎЙИЕФжївЊГЩЗжЪЧЬМЫсИЦЁЂСђЫсИЦЁЂЧтбѕЛЏУОЁЂЬњатЁЂЖўбѕЛЏЙшЕШЁЃЫЎЙИашМАЪБЧхЯДГ§ШЅЁЃЧхЯДСїГЬШчЯТЃК
Ђё.МгШыNaOHКЭNa2CO3ЛьКЯвКЃЌМгШШЃЌНўХнЪ§аЁЪБЃЛ
Ђђ.ЗХГіЯДЕгЗЯвКЃЌЧхЫЎГхЯДЙјТЏЃЌМгШыЯЁбЮЫсКЭЩйСПNaFШмвКЃЌНўХнЃЛ
Ђѓ.ЯђЯДЕгвКжаМгШыNa2SO3ШмвКЃЛ
Ђє.ЧхЯДДяБъЃЌгУNaNO2ШмвКЖлЛЏЙјТЏЁЃ
ЂйгУЯЁбЮЫсШмНтЬМЫсИЦЕФРызгЗНГЬЪНЪЧ_____________________________ЁЃ
ЂквбжЊЃК25 ЁцЪБгаЙиЮяжЪЕФШмЖШЛ§
ЮяжЪ | CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
Ksp | 2.8ЁС10Ѓ9 | 9.1ЁС10Ѓ6 | 1.8ЁС10Ѓ11 | 6.8ЁС10Ѓ6 |
ИљОнЪ§ОнЃЌНсКЯЛЏбЇЦНКтдРэНтЪЭЧхЯДCaSO4ЕФЙ§ГЬ________________ЁЃЃЈгУШмНтЦНКтБэДяЪНКЭБивЊЕФЮФзжа№ЪіМгвдЫЕУїЃЉЃЛдкВНжшЂёНўХнЙ§ГЬжаЛЙЛсЗЂЩњЗДгІMgCO3(s)ЃЋ2OHЃ(aq)![]() Mg(OH)2(s)ЃЋCO32-(aq)ЃЌИУЗДгІЕФЦНКтГЃЪ§KЃН________(БЃСєСНЮЛгааЇЪ§зж)ЁЃ
Mg(OH)2(s)ЃЋCO32-(aq)ЃЌИУЗДгІЕФЦНКтГЃЪ§KЃН________(БЃСєСНЮЛгааЇЪ§зж)ЁЃ
ЂлВНжшЂѓжаЃЌМгШыNa2SO3ШмвКЕФФПЕФЪЧ_______________________________ЁЃ