��Ŀ����
����Ŀ��п������BaSO4��ZnS�Ļ���ZnS������ˮ��ij���������ؾ�ʯΪԭ���Ʊ�п���ף����������л����������������Ҫ�ɷ�ΪCO2��CO������SO2���������ȣ���Ϊ��ֹ��Ⱦ���������ԭ�������ʣ������в��������������̣�
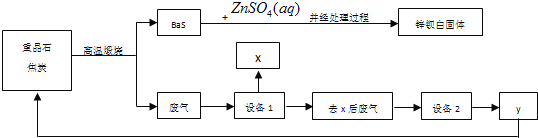
������������̻ش��������⣺
(1)�ؾ�ʯ�Ļ�ѧʽΪ_______��п�������ڵ���_____ɫ��dzɫ���ᡣ
(2)��������![]() �IJ���Ϊ����_________����ϴ�ӣ���______________��
�IJ���Ϊ����_________����ϴ�ӣ���______________��
(3)�豸1����ȴ�����������豸1ǰ��ĵ����ܹ�������խ������ʹ�����ܶ�������ԭ����__________________________________��
(4)����п�����к���S2-�ķ�����________________________________________��
(5)�豸2�����õ�ϴ�Ӽ���NaOH��Һ����y�Ļ�ѧʽΪ________��д���豸2�з�����Ӧ�����ӷ���ʽ��___________________________________��_________________________________��
(6)����_______��������Һ�е����Σ����з���ǰ����Ҫ��õ���______________________��
(7)�Ʊ������еõ�����ǣ����˿������������⣬��������;Ϊ___________������дһ�֣���
���𰸡�BaSO4 �� ���� ���� ��������ȴ����˹��� ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������� CO SO2+2OH-=SO32-+H2O CO2+2OH-=CO32-+H2O �ᾧ �����ʵ��ܽ�����¶ȵı仯��� ����ҩ��ũҩ����Ƥ������
��������
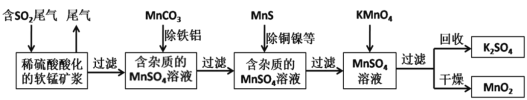
���ݹ�ҵ���̣��ؾ�ʯ��̼����������ԭ��Ӧ������ԭ��������������������п�������ֽⷴӦ�ɵ����ᱵ����п��п���ף���������Ҫ�ɷ�ΪCO2��CO������SO2���������ȣ������豸1��X���豸1����ȴ��������XӦΪS�����豸1ǰ��ĵ����ܹ�������խ����������ȴ����˹��壬��ʹ�����ܶ�����ȥ���ķ�����Ҫ��CO2��CO������SO2�������豸2���豸2�����õ�ϴ�Ӽ���NaOH��Һ��CO2��SO2�������������գ���õ���YΪCO��CO��ѭ�����ã���ԭ�ؾ�ʯ��
��1�����ᱵ���������ؾ�ʯ���ؾ�ʯ�Ļ�ѧʽΪBaSO4�����ᱵ����п���ǰ�ɫ���壬п�������ڵ��ư�ɫ��dzɫ���ᣬ
�ʴ�Ϊ��BaSO4���ף�
��2�����������ǽ���������п�����÷ֽⷴӦ�ɵ����ᱵ����п�����õ����壬����ʵ�鲽��Ϊ���ٹ��ˣ���ϴ�ӣ��۸��
�ʴ�Ϊ�����ˣ����
��3�����ݷ�����֪��ԭ������������ȴ����˹��壬
�ʴ�Ϊ����������ȴ����˹��壻
��4������S2�������ʿ�֪������п�����к���S2���ķ�����ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������ӣ�
�ʴ�Ϊ��ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������ӣ�
��5��CO2��SO2�������������գ���y�Ļ�ѧʽΪCO���豸2�з�����Ӧ�����ӷ���ʽ��SO2+2OH��=SO32��+H2O��CO2+2OH��=CO32��+H2O��
�ʴ�Ϊ��CO��SO2+2OH��=SO32��+H2O��CO2+2OH��=CO32��+H2O��
��6���豸2��Һ�е�����Ϊ̼���ơ��������Ƶȣ�����������ˮ�ģ��ɸ������ǵ��ܽ�����¶ȵĹ�ϵ���ýᾧ��������з���ǰ��������Ҫ��õ��Ǹ����ʵ��ܽ�����¶ȵı仯�����
�ʴ�Ϊ���ᾧ�������ʵ��ܽ�����¶ȵı仯�����
��7�����������;��֪���Ʊ������еõ�����ǣ����˿������������⣬����������������ҩ��ũҩ�Լ���Ƥ�����ȣ�
�ʴ�Ϊ��������ҩ��ũҩ�Լ���Ƥ�����ȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�