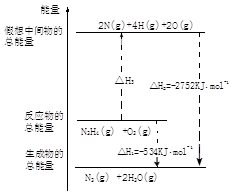��Ŀ����
ijѧϰС��̽��Ũ��ϡ���������Ե����ǿ��������ͼװ�ý���ʵ�飨�г���������ȥ����

��ѡҩƷ��ϡ���ᡢŨ���ᡢŨ���ᡢNaOH��Һ������ˮ
�������ϣ�
A��Ũ�����ܽ�NO������NO2����ϡ���������NO��
B������������Һ����NO��Ӧ������NO2��Ӧ2NO2 + 2NaOH = NaNO3 + NaNO2 +H2O
��1�����з�����Ӧ�����ӷ���ʽ�� ��
��2�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
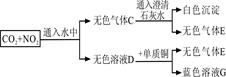
��3��װ�â�~����ʢ�ŵ�ҩƷ�ֱ��Ǣ� ���� ���� ���� ��
��4��II�IJ����� ��Ŀ���� ��
��5����ͬѧ�ó����������ݵ�ʵ�������� ��

��ѡҩƷ��ϡ���ᡢŨ���ᡢŨ���ᡢNaOH��Һ������ˮ
�������ϣ�
A��Ũ�����ܽ�NO������NO2����ϡ���������NO��
B������������Һ����NO��Ӧ������NO2��Ӧ2NO2 + 2NaOH = NaNO3 + NaNO2 +H2O
| ʵ����� | ʵ������ |
| I������װ�õ������� | |
| II������ | |
| III����Һ©����������Ũ���Ỻ��������ƿ�У��رջ����� | ���в��������ĺ���ɫ���壬����ɫ�����ڢ��б�Ϊ��ɫ������ͨ���ۺ���ȻΪ��ɫ��ͨ���ܺ��Ϊ����ɫ |
��1�����з�����Ӧ�����ӷ���ʽ�� ��
��2�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3��װ�â�~����ʢ�ŵ�ҩƷ�ֱ��Ǣ� ���� ���� ���� ��
��4��II�IJ����� ��Ŀ���� ��
��5����ͬѧ�ó����������ݵ�ʵ�������� ��
��1��Cu+4H++2NO3��== Cu2++2NO2��+2H2O
��2��3NO2+ H2O == 2HNO3+ NO
��3������ˮ��ϡ���ᡢŨ���ᡢ����������Һ
��4�����ɼУ�ͨһ��ʱ��N2���رյ��ɼ� �Ͼ�װ���еĿ�������������
��5������Һ���Ϸ�Ϊ��ɫ������Һ���Ϸ���Ϊ����ɫ
��2��3NO2+ H2O == 2HNO3+ NO
��3������ˮ��ϡ���ᡢŨ���ᡢ����������Һ
��4�����ɼУ�ͨһ��ʱ��N2���رյ��ɼ� �Ͼ�װ���еĿ�������������
��5������Һ���Ϸ�Ϊ��ɫ������Һ���Ϸ���Ϊ����ɫ
�����������1���ڢ���Cu��Ũ���ᷢ����Ӧ��Cu+4HNO3(Ũ)=Cu(NO3)2+NO2��+2H2O���ӷ���ʽ��Cu+4H++2NO3��== Cu2++2NO2��+2H2O.��2�����з�����Ӧ�Ļ�ѧ����ʽ��3NO2+ H2O == 2HNO3+ NO����3��װ�â�~����ʢ�ŵ�ҩƷ�ֱ��Ǣ�����ˮ����ϡ���ᡢ��Ũ���ᡢ������������Һ����4������װ�õ������Ժ���е�II�IJ����Ǵ��ɼУ�ͨһ��ʱ��N2���رյ��ɼУ�Ŀ���ǸϾ�װ���еĿ�������������ʹ����ϵͳ���ڶ��Ի����У���ֹ�����е������ѷ�Ӧ������NO������Ӱ��ʵ�������жϡ���5�����з�����Ӧ��Cu+4HNO3(Ũ)=Cu(NO3)2+NO2��+2H2O�����з�����Ӧ3NO2+ H2O == 2HNO3+ NO,�۲���Ӧ�����з�����ӦNO+2HNO3(Ũ)= 3NO2��+H2O�����з�����Ӧ2NO2 + 2NaOH = NaNO3 + NaNO2 +H2O.��˸�ͬѧ�ó����������ݵ�ʵ�������Ǣ���Һ���Ϸ�Ϊ��ɫ������Һ���Ϸ���Ϊ����ɫ��

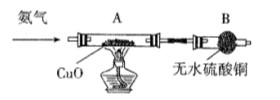
��ϰ��ϵ�д�
�����Ŀ