��Ŀ����
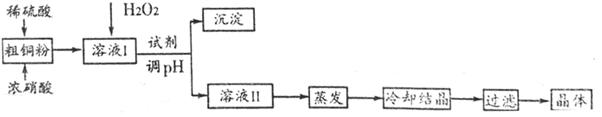
����������Ҫ��ҵ���ϡ���ҵ��ͨ���Է���м�����ʲ����ᷴӦ��Ϊԭ���Ʊ�FeCO3���ٽ���������ȡ����������ҵ�Ʊ�FeCO3���������£�
�ش��������⣺
��1��������������� ��
��2��д������FeCO3���������ӷ���ʽ ��
��3����Щͬѧ��Ϊ��Һ������Ԫ�غ�������KMnO4��Һ���ⶨ��5Fe2++MnO-4+8H+=5Fe3++Mn2++4H2O����
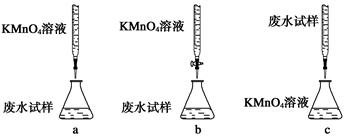
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
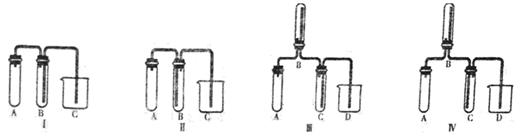
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�

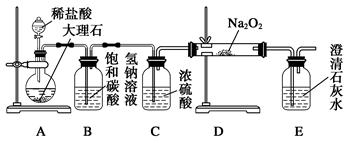
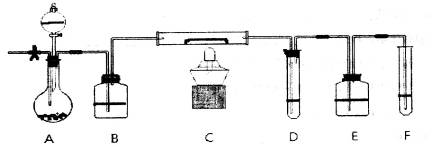
��4�����������õ���Na2CO3�ڹ�ҵ�����г���������NaCl��ijУ��ѧ����������ͼ��ʾװ�����ⶨNa2CO3�ĺ�����
��Ҫ����Na2CO3�����ʵĴ��ڣ�ѡ�������Լ��е� ��ѡ����ţ���
a������������Һ b��ϡ���� c�����������Һ d����������Һ
�ڼ���װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ���ã��� ��˵��װ�ò�©����
��װ��A�е��Լ� ��װ��C������ ��
������ʵ��װ�ô�������ȱ�ݣ���ȱ�ݵ��²ⶨ���ƫ�ߣ���ȱ��Ϊ ��
�ش��������⣺
��1��������������� ��
��2��д������FeCO3���������ӷ���ʽ ��
��3����Щͬѧ��Ϊ��Һ������Ԫ�غ�������KMnO4��Һ���ⶨ��5Fe2++MnO-4+8H+=5Fe3++Mn2++4H2O����
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�

��4�����������õ���Na2CO3�ڹ�ҵ�����г���������NaCl��ijУ��ѧ����������ͼ��ʾװ�����ⶨNa2CO3�ĺ�����

��Ҫ����Na2CO3�����ʵĴ��ڣ�ѡ�������Լ��е� ��ѡ����ţ���
a������������Һ b��ϡ���� c�����������Һ d����������Һ
�ڼ���װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ���ã��� ��˵��װ�ò�©����
��װ��A�е��Լ� ��װ��C������ ��
������ʵ��װ�ô�������ȱ�ݣ���ȱ�ݵ��²ⶨ���ƫ�ߣ���ȱ��Ϊ ��
(14��)
��1������(2��)
��2��Fe2+ +HCO- 3 = FeCO 3��+ CO2 +H2O ( 2��)
��3����250mL����ƿ(1��) ��b(1��)
��4����bd(2��)
��©�������Լ�ƿ�е�Һ���ٱ仯��©���е�Һ�治���½�(2��)
��NaOH��Һ(1��) ����(1��)�����������𰸾��÷֣�
����BC֮��ȱ������HCl��װ�á���Dװ�ú�ȱ��ʢ�м�ʯ�ҵĸ���ܣ��������1����2�֣����������𰸾��÷֣�
��1������(2��)
��2��Fe2+ +HCO- 3 = FeCO 3��+ CO2 +H2O ( 2��)
��3����250mL����ƿ(1��) ��b(1��)
��4����bd(2��)
��©�������Լ�ƿ�е�Һ���ٱ仯��©���е�Һ�治���½�(2��)
��NaOH��Һ(1��) ����(1��)�����������𰸾��÷֣�
����BC֮��ȱ������HCl��װ�á���Dװ�ú�ȱ��ʢ�м�ʯ�ҵĸ���ܣ��������1����2�֣����������𰸾��÷֣�
���������
��1����Һ�����ȡ���˲�����
��2�����������Fe2+ +HCO- 3 = FeCO 3��+ CO2 +H2O�������⡣
��3��������һ�����ʵ���Ũ����Һ��Ҫ����ƿ������Ϊ250mL����Fe2+��KMnO4��Һ��Ϊ������KMnO4��Һǿ�����ԣ�ʹ����ʽ�ζ��ܡ�
��4��
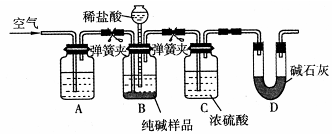
��Na2CO3�к�������NaCl������ϡ�����ữ����������Һ��
�ڼ�������ԣ���������ѹǿ�©�������Լ�ƿ�е�Һ���ٱ仯��©���е�Һ�治���½����ɣ�
����NaOH��Һ���տ����ж�����̼����ֹ�������ţ���Ũ�����ȥ������ˮ�֡�
�ܿɼ�BC֮��ȱ�ٳ�ȥHCl��װ�ã����Ҵ����гɷ�Ҳ�����D��

��ϰ��ϵ�д�
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ


 ����μ��麬��Fe2+____________________________________��
����μ��麬��Fe2+____________________________________��