��Ŀ����
��ѧʵ�����о��������ʵĻ�����
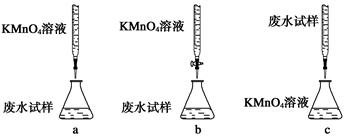
(1)�����й�ʵ�������������ݺ�������________(�����)��
a��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b���ø����pH��ֽ�ⶨŨ�����pH
c���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
(2)ij��ˮ��Ʒ�к���һ������Na����CO32-��SO32-��ij�о�С�����ⶨ����SO32-��Ũ�ȡ�
ʵ�鷽����
��.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ�е����ʣ����ˣ�ȡ��Һ��
��.��ȷ��ȡ20.00 mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol/L KMnO4(H2SO4�ữ)��Һ���еζ���
��.��¼���ݣ����㡣
�����еζ���ʽ�У����������(�гֲ��ѷ���ȥ)______(����ĸ���)��
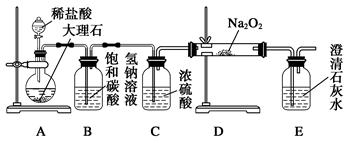
�ڵζ������У��йط�Ӧ�����ӷ���ʽ��__________________________________��
(3)ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ���ձ�ȡ��������ˮ���������ڣ����ձ��еμ� 1 mol/L��FeCl3��Һ���������ò��������裬�����Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ������������������������Ϊ�ɹ��Ƶ�Fe(OH)3���������������_________��
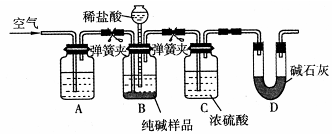
(4)����ͼװ�ý���CO2���ʵ��й�ʵ�顣
�Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ����:
_______________________ __________��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ��
____________________ ___________��
(1)�����й�ʵ�������������ݺ�������________(�����)��
a��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b���ø����pH��ֽ�ⶨŨ�����pH
c���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
(2)ij��ˮ��Ʒ�к���һ������Na����CO32-��SO32-��ij�о�С�����ⶨ����SO32-��Ũ�ȡ�
ʵ�鷽����
��.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ�е����ʣ����ˣ�ȡ��Һ��
��.��ȷ��ȡ20.00 mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol/L KMnO4(H2SO4�ữ)��Һ���еζ���
��.��¼���ݣ����㡣
�����еζ���ʽ�У����������(�гֲ��ѷ���ȥ)______(����ĸ���)��

�ڵζ������У��йط�Ӧ�����ӷ���ʽ��__________________________________��
(3)ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ���ձ�ȡ��������ˮ���������ڣ����ձ��еμ� 1 mol/L��FeCl3��Һ���������ò��������裬�����Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ������������������������Ϊ�ɹ��Ƶ�Fe(OH)3���������������_________��
(4)����ͼװ�ý���CO2���ʵ��й�ʵ�顣

�Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ����:
_______________________ __________��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ��
____________________ ___________��
(1)c(2)��b����2MnO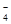 +5SO
+5SO +6H+��2Mn2++5SO
+6H+��2Mn2++5SO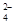 +3H2O
+3H2O
(3)���赼�½���۳���Һ������ĺ��ɫ(�������ɫ)
(4)�ٳ�ȥHCl�������� ��CaCO3(s) Ca2+(aq)+CO
Ca2+(aq)+CO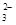 (aq)
(aq)
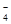 +5SO
+5SO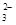 +6H+��2Mn2++5SO
+6H+��2Mn2++5SO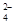 +3H2O
+3H2O(3)���赼�½���۳���Һ������ĺ��ɫ(�������ɫ)
(4)�ٳ�ȥHCl�������� ��CaCO3(s)
 Ca2+(aq)+CO
Ca2+(aq)+CO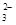 (aq)
(aq)�����������1��a�����������ܺ�ˮ������Ӧ��ʵ�鲻��������������CuSO4��5H2O����ⶨ�ᾧˮ����������a����ȷ��
b��Ũ���������ˮ�ԣ������ø����pH��ֽ�ⶨŨ�����pH��b����ȷ��
c����Ͳ���Զ�����0.1ml�������ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ��c��ȷ����ѡc��
��2�������Ը��������ҺӦ�÷�����ʽ�ζ����У�a�����Ը��������Һ���ڼ�ʽ�ζ����У����a����ȷ��b��ȷ��Ӧ���ø��������Һ�ζ���ˮ��ѡ��c����ȷ����ѡb��
�ڸ�����ؾ���ǿ�����ԣ��ܰ�SO
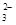 ���������Եζ������У��йط�Ӧ�����ӷ���ʽ��2MnO
���������Եζ������У��йط�Ӧ�����ӷ���ʽ��2MnO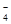 +5SO
+5SO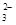 +6H+��2Mn2++5SO
+6H+��2Mn2++5SO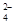 +3H2O��
+3H2O����3���������Ȼ���������Һ�����ˮ���Ʊ������������壬����ֱ��Һ���Ϊ���ĺ��ɫֹͣ���ȣ����貣�������衣���Ը�ʵ��ʧ�ܵ�ԭ���ǽ��赼�½���۳���
��4���������ӷ������ɵ�CO2�к����Ȼ������壬���Ա���̼��������Һ�������dz�ȥHCl�������塣
��̼��������������ʣ������ܽ�ƽ�⣬����ʽ��CaCO3(s)
 Ca2+(aq)+CO
Ca2+(aq)+CO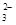 (aq)��
(aq)����������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ա����������ڸ߿�������һ�����ǵ������⣬��һ�����dz������ۺ�ʵ�������У���ǰ����˵�������Ȼ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ