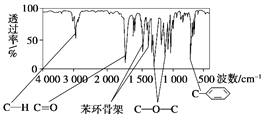
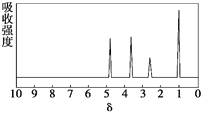
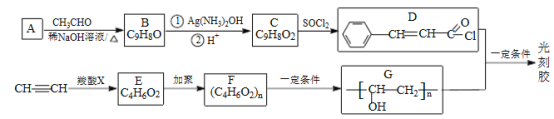
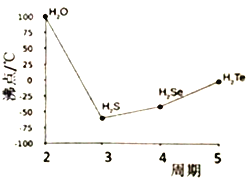
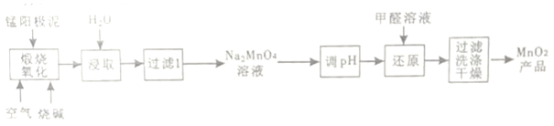
��Ŀ����
����Ŀ����.����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�

��1������¯��Al2(SO4)3����Ƿ�Ӧ����һ����ʹƷ����Һ��ɫ�����ʺ�һ�־������Ե�������д���÷�Ӧ��ѧ����ʽ______________________________________________________��
��2���Լ�X���Ϊ�����Լ��е�_________
A.NaOH B.CO2 C.���� D.��ˮ
�����ܽ�ʱ��������Ӧ�����ӷ���ʽΪ_____________________________________��
��3��ĸҺ��������Ҫ�ɷֵĻ�ѧʽΪ__________����Һ����pH���ˡ�ϴ�ӿɵ�Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������_______________________________________________��
��. ��100mlij����Һ�����ܺ������������е���������Mg2����Cu2����Fe2����Al3����NH4����K+��HCO3����SO42����������һ�ֵ���ɫ��ĩ״��������ʱ���д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������������0.6mol����ɫ��ĩʱ�����ռ���0.8mol������壬�Ҵ�ʱ���ɵij�����ࡣ�˺�������뵭��ɫ��ĩʱ�����������٣�������0.65mol��ĩ��������0.3mol������0.2mol���ټ����ĩ�����Ͳ��ټ��١��ɴ�ʵ�����������жϣ�
��4������ɫ��ĩΪ_________________��
��5����Һ�п϶���__________________������
��6����Һ�������Ӱ������ٵ��������ʵ���֮��Ϊ______________��H����û��ȷ�������ӳ��⣩��
���𰸡� 2Al2��SO4��3+3S![]() 2Al2O3+9SO2�� A Al2O3+2OH-=2AlO2-+H2O K2SO4��Na2SO4 ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ� Na2O2 Mg2����Al3����NH4����SO42�� 1:2:5
2Al2O3+9SO2�� A Al2O3+2OH-=2AlO2-+H2O K2SO4��Na2SO4 ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ� Na2O2 Mg2����Al3����NH4����SO42�� 1:2:5
����������.�������������ʯ����ˮ��������һ���գ��õ������Ͼ�����������Һ���ɱ���Խϵ͵�ǿ���ȡ���ˣ�������ҪΪ�������ȡ�����Һ�м����������pH�õ������������������˺��ĸҺ�к��������ƺ�����صȵȡ�
��1������¯��Al2(SO4)3����Ƿ�Ӧ���ɵ���ʹƷ����Һ��ɫ�������Ƕ���������һ�־������Ե����������������÷�Ӧ��ѧ����ʽΪ2Al2(SO4)3+3S![]() 2Al2O3+9SO2����
2Al2O3+9SO2����
��2���Լ�X���Ϊ�����Լ�ΪNaOH��ѡA �������ܽ�ʱ��������Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
��3��ĸҺ��������Ҫ�ɷֵĻ�ѧʽΪK2SO4��Na2SO4����Һ����pH���ˡ�ϴ�ӿɵ�Al(OH)3������֤��������ϴ�Ӹɾ��ķ����ǣ�����ϴ��Һ���Ƿ�����������ӣ�ʵ���������������ȡ����ϴ��Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ������������ϴ�Ӹɾ���
��. ��100mlij����Һ�����ܺ������������е������֣�Mg2����Cu2����Fe2����Al3������K+��HCO3������������һ�ֵ���ɫ��ĩ״��������ʱ���д̼�����ζ�Ļ������ų����õ���ɫ��ĩ״��������һ���ǹ������ƣ��������Ϊ��������������һ������NH4����ͬʱ���ɰ�ɫ�������ó���������������þ�������������϶�û��Cu2����Fe2����������0.6mol����ɫ��ĩʱ�����ռ���0.8mol������壬��������0.3mol O2��0.5molNH3����ʱ���ɵij�����ࡣ�˺�������뵭��ɫ��ĩʱ�����������٣�������0.65mol��ĩ��������0.3mol������0.2mol���ټ����ĩ�����Ͳ��ټ�������һ����0.2 mol Mg2����0.1mol Al3������Ϊ̼����������������Ӳ��ܴ������棬����һ��û��HCO3�����ɵ���غ�֪��һ����SO42����
��4������ɫ��ĩΪNa2O2��
��5����Һ�п϶���Mg2����Al3����NH4����SO42�����ӣ�
��6��0.65mol Na2O2��ˮ��Ӧ������1.3mol OH-��0.2 mol Mg2����0.1mol Al3��������0.4 mol OH-������һ����0.5mol NH4�������������0.5mol OH-�����ԣ�H����û��ȷ�������ӳ�������Һ�������Ӱ������ٵ��������ʵ���֮��Ϊ1:2:5��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�